Mitochondrial ROS in Slc4a11 KO Corneal Endothelial Cells Lead to ER Stress
- PMID: 35557943
- PMCID: PMC9086159
- DOI: 10.3389/fcell.2022.878395
Mitochondrial ROS in Slc4a11 KO Corneal Endothelial Cells Lead to ER Stress
Abstract
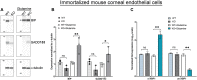
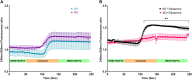
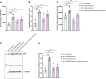
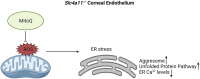
Recent studies from Slc4a11 -/- mice have identified glutamine-induced mitochondrial dysfunction as a significant contributor toward oxidative stress, impaired lysosomal function, aberrant autophagy, and cell death in this Congenital Hereditary Endothelial Dystrophy (CHED) model. Because lysosomes are derived from endoplasmic reticulum (ER)-Golgi, we asked whether ER function is affected by mitochondrial ROS in Slc4a11 KO corneal endothelial cells. In mouse Slc4a11 -/- corneal endothelial tissue, we observed the presence of dilated ER and elevated expression of ER stress markers BIP and CHOP. Slc4a11 KO mouse corneal endothelial cells incubated with glutamine showed increased aggresome formation, BIP and GADD153, as well as reduced ER Ca2+ release as compared to WT. Induction of mitoROS by ETC inhibition also led to ER stress in WT cells. Treatment with the mitochondrial ROS quencher MitoQ, restored ER Ca2+ release and relieved ER stress markers in Slc4a11 KO cells in vitro. Systemic MitoQ also reduced BIP expression in Slc4a11 KO endothelium. We conclude that mitochondrial ROS can induce ER stress in corneal endothelial cells.
Keywords: ERAD (ER associated protein degradation); MitoQ; ROS—reactive oxygen species; SLC4A11 ammonia transporter; corneal endothelial cells; er stress.
Copyright © 2022 Shyam, Ogando and Bonanno.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Mitochondrial ROS Induced Lysosomal Dysfunction and Autophagy Impairment in an Animal Model of Congenital Hereditary Endothelial Dystrophy.Invest Ophthalmol Vis Sci. 2021 Sep 2;62(12):15. doi: 10.1167/iovs.62.12.15. Invest Ophthalmol Vis Sci. 2021. PMID: 34533563 Free PMC article.
-
Ammonia sensitive SLC4A11 mitochondrial uncoupling reduces glutamine induced oxidative stress.Redox Biol. 2019 Sep;26:101260. doi: 10.1016/j.redox.2019.101260. Epub 2019 Jun 23. Redox Biol. 2019. PMID: 31254733 Free PMC article.
-
Antioxidant MitoQ increases viability of human corneal endothelial cells with congenital hereditary endothelial dystrophy-associated SLC4A11 mutations.Ophthalmic Genet. 2025 Apr;46(2):166-173. doi: 10.1080/13816810.2025.2450455. Epub 2025 Jan 20. Ophthalmic Genet. 2025. PMID: 39834031
-
The H+ Transporter SLC4A11: Roles in Metabolism, Oxidative Stress and Mitochondrial Uncoupling.Cells. 2022 Jan 7;11(2):197. doi: 10.3390/cells11020197. Cells. 2022. PMID: 35053313 Free PMC article. Review.
-
SLC4A11 and the Pathophysiology of Congenital Hereditary Endothelial Dystrophy.Biomed Res Int. 2015;2015:475392. doi: 10.1155/2015/475392. Epub 2015 Sep 16. Biomed Res Int. 2015. PMID: 26451371 Free PMC article. Review.
Cited by
-
SLC4A11 Revisited: Isoforms, Expression, Functions, and Unresolved Questions.Biomolecules. 2025 Jun 16;15(6):875. doi: 10.3390/biom15060875. Biomolecules. 2025. PMID: 40563515 Free PMC article. Review.
-
Potential Theranostic Roles of SLC4 Molecules in Human Diseases.Int J Mol Sci. 2023 Oct 13;24(20):15166. doi: 10.3390/ijms242015166. Int J Mol Sci. 2023. PMID: 37894847 Free PMC article. Review.
-
Endoplasmic reticulum stress: molecular mechanism and therapeutic targets.Signal Transduct Target Ther. 2023 Sep 15;8(1):352. doi: 10.1038/s41392-023-01570-w. Signal Transduct Target Ther. 2023. PMID: 37709773 Free PMC article. Review.
-
Updates on congenital hereditary endothelial dystrophy.Taiwan J Ophthalmol. 2023 Nov 28;13(4):405-416. doi: 10.4103/tjo.TJO-D-23-00135. eCollection 2023 Oct-Dec. Taiwan J Ophthalmol. 2023. PMID: 38249503 Free PMC article. Review.
-
MitoQ relieves mitochondrial dysfunction in UVA and cigarette smoke-induced Fuchs endothelial corneal dystrophy.Exp Eye Res. 2024 Oct;247:110056. doi: 10.1016/j.exer.2024.110056. Epub 2024 Aug 22. Exp Eye Res. 2024. PMID: 39179169
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

