Electrodegradation of 2,4-dichlorophenoxyacetic acid herbicide from aqueous solution using three-dimensional electrode reactor with G/β-PbO2 anode: Taguchi optimization and degradation mechanism determination
- PMID: 35558020
- PMCID: PMC9090970
- DOI: 10.1039/c8ra08471h
Electrodegradation of 2,4-dichlorophenoxyacetic acid herbicide from aqueous solution using three-dimensional electrode reactor with G/β-PbO2 anode: Taguchi optimization and degradation mechanism determination
Abstract
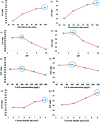
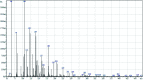
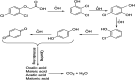
This study aimed to investigate the electro-degradation of 2,4-dichlorophenoxyacetic acid (2,4-D) from aqueous solution using two and three-dimensional electrode (2D and 3D) reactors with graphite(G)/β-PbO2 anode. To increase the degradation efficiency, affecting parameters on the electro-degradation process were investigated and optimized by adopting the Taguchi design of experiments approach. The structure, morphology and electrochemical properties of the electrodes were studied by X-ray diffraction (XRD), scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDX), linear sweep voltammetry and cyclic voltammograms. The controllable factors, i.e., electrolysis time, 2,4-D initial concentration, solution pH and current density (j) were optimized. Under optimum conditions, the 2,4-D degradation efficiency was 75.6% using 2D and 93.5% using 3D electrode processes. The percentage contribution of each controllable factor was also determined. The pH of the solution was identified as the most influential factor, and its percentage contribution value was up to 39.9% and 40.4% for 2D and 3D electrode processes, respectively. Considering the parameters of the kinetics, it was found that the degradation of 2,4-D and removal of COD using the G/β-PbO2 electrode obey the pseudo-first order kinetics. In addition, the mineralization pathway of 2,4-D at G/β-PbO2 electrode was proposed. The results also demonstrated that the 3D electrode process with G/β-PbO2 anode can be considered as a useful method for degradation and mineralization of 2,4-D herbicides from aqueous solution.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources

