A critical review on arsenic removal from water using iron-based adsorbents
- PMID: 35558047
- PMCID: PMC9091186
- DOI: 10.1039/c8ra08512a
A critical review on arsenic removal from water using iron-based adsorbents
Abstract
Intensive research efforts have been pursued to remove arsenic (As) contamination from water with an intention to provide potable water to millions of people living in different countries. Recent studies have revealed that iron-based adsorbents, which are non-toxic, low cost, and easily accessible in large quantities, offer promising results for arsenic removal from water. This review is focused on the removal of arsenic from water using iron-based materials such as iron-based nanoparticles, iron-based layered double hydroxides (LDHs), zero-valent iron (ZVI), iron-doped activated carbon, iron-doped polymer/biomass materials, iron-doped inorganic minerals, and iron-containing combined metal oxides. This review also discusses readily available low-cost adsorbents such as natural cellulose materials, bio-wastes, and soils enriched with iron. Details on mathematical models dealing with adsorption, including thermodynamics, kinetics, and mass transfer process, are also discussed. For elucidating the adsorption mechanisms of specific adsorption of arsenic on the iron-based adsorbent, X-ray photoelectron spectroscopy (XPS) and X-ray absorption spectroscopy (XAS) are frequently used. Overall, iron-based adsorbents offer significant potential towards developing adsorbents for arsenic removal from water.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
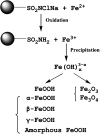



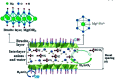
References
-
- Siddiqui S. I. Chaudhry S. A. Process Saf. Environ. Prot. 2017;111:592–626. doi: 10.1016/j.psep.2017.08.009. - DOI
-
- Siddiqui S. I. Chaudhry S. A. Curr. Environ. Eng. 2017;4:81–102. doi: 10.2174/2212717804666161214143715. - DOI
-
- Jomova K. Jenisova Z. Feszterova M. et al. . J. Appl. Toxicol. 2011;31:95–107. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

