Rapid Inflammasome Activation Is Attenuated in Post-Myocardial Infarction Monocytes
- PMID: 35558073
- PMCID: PMC9090500
- DOI: 10.3389/fimmu.2022.857455
Rapid Inflammasome Activation Is Attenuated in Post-Myocardial Infarction Monocytes
Abstract
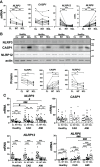
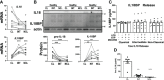
Inflammasomes are crucial gatekeepers of the immune response, but their maladaptive activation associates with inflammatory pathologies. Besides canonical activation, monocytes can trigger non-transcriptional or rapid inflammasome activation that has not been well defined in the context of acute myocardial infarction (AMI). Rapid transcription-independent inflammasome activation induced by simultaneous TLR priming and triggering stimulus was measured by caspase-1 (CASP1) activity and interleukin release. Both classical and intermediate monocytes from healthy donors exhibited robust CASP1 activation, but only classical monocytes produced high mature interleukin-18 (IL18) release. We also recruited a limited number of coronary artery disease (CAD, n=31) and AMI (n=29) patients to evaluate their inflammasome function and expression profiles. Surprisingly, monocyte subpopulations isolated from blood collected during percutaneous coronary intervention (PCI) from AMI patients presented diminished CASP1 activity and abrogated IL18 release despite increased NLRP3 gene expression. This unexpected attenuated rapid inflammasome activation was accompanied by a significant increase of TNFAIP3 and IRAKM expression. Moreover, TNFAIP3 protein levels of circulating monocytes showed positive correlation with high sensitive troponin T (hsTnT), implying an association between TNFAIP3 upregulation and the severity of tissue injury. We suggest this monocyte attenuation to be a protective phenotype aftermath following a very early inflammatory wave in the ischemic area. Damage-associated molecular patterns (DAMPs) or other signals trigger a transitory negative feedback loop within newly recruited circulating monocytes as a mechanism to reduce post-injury tissue damage.
Keywords: IRAKM; TNFAIP3 (A20); acute myocardial infarction; inflammasome; interleukin 18; monocytes; refractory behavior.
Copyright © 2022 Giral, Franke, Moobed, Müller, Lübking, James, Hartung, Kuschnerus, Meteva, Seppelt, Jakob, Klingenberg, Kränkel, Leistner, Zeller, Blankenberg, Zimmermann, Haghikia, Lüscher, Akalin, Landmesser and Kratzer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

