Microglia in the Neuroinflammatory Pathogenesis of Alzheimer's Disease and Related Therapeutic Targets
- PMID: 35558075
- PMCID: PMC9086828
- DOI: 10.3389/fimmu.2022.856376
Microglia in the Neuroinflammatory Pathogenesis of Alzheimer's Disease and Related Therapeutic Targets
Abstract
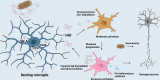
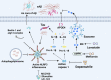
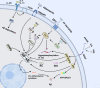
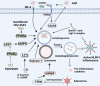
Alzheimer's disease (AD) is the most prevalent neurodegenerative disease worldwide, characterized by progressive neuron degeneration or loss due to excessive accumulation of β-amyloid (Aβ) peptides, formation of neurofibrillary tangles (NFTs), and hyperphosphorylated tau. The treatment of AD has been only partially successful as the majority of the pharmacotherapies on the market may alleviate some of the symptoms. In the occurrence of AD, increasing attention has been paid to neurodegeneration, while the resident glial cells, like microglia are also observed. Microglia, a kind of crucial glial cells associated with the innate immune response, functions as double-edge sword role in CNS. They exert a beneficial or detrimental influence on the adjacent neurons through secretion of both pro-inflammatory cytokines as well as neurotrophic factors. In addition, their endocytosis of debris and toxic protein like Aβ and tau ensures homeostasis of the neuronal microenvironment. In this review, we will systematically summarize recent research regarding the roles of microglia in AD pathology and latest microglia-associated therapeutic targets mainly including pro-inflammatory genes, anti-inflammatory genes and phagocytosis at length, some of which are contradictory and controversial and warrant to further be investigated.
Keywords: alzheimer’s disease; anti-neuroinflammation; microglial cells; molecular therapy; neuroinflammation.
Copyright © 2022 Cai, Liu, Wang, Sun and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

