Role of Programmed Cell Death Protein-1 and Lymphocyte Specific Protein Tyrosine Kinase in the Aryl Hydrocarbon Receptor- Mediated Impairment of the IgM Response in Human CD5+ Innate-Like B Cells
- PMID: 35558082
- PMCID: PMC9088000
- DOI: 10.3389/fimmu.2022.884203
Role of Programmed Cell Death Protein-1 and Lymphocyte Specific Protein Tyrosine Kinase in the Aryl Hydrocarbon Receptor- Mediated Impairment of the IgM Response in Human CD5+ Innate-Like B Cells
Abstract
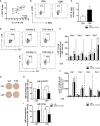
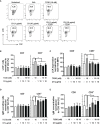
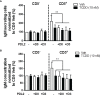
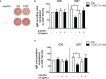
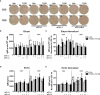
Innate-like B cells (ILBs) are a heterogeneous population B cells which participate in innate and adaptive immune responses. This diverse subset of B cells is characterized by the expression of CD5 and has been shown to secrete high levels of immunoglobulin M (IgM) in the absence of infection or vaccination. Further, CD5+ ILBs have been shown to express high basal levels of lymphocyte specific protein tyrosine kinase (LCK) and programmed cell death protein-1 (PD-1), which are particularly sensitive to stimulation by interferon gamma (IFNγ). Previous studies have demonstrated that activation of the aryl hydrocarbon receptor (AHR), a cytosolic ligand-activated transcription factor, results in suppressed IgM responses and is dependent on LCK. A recent study showed that CD5+ ILBs are particularly sensitive to AHR activation as evidenced by a significant suppression of the IgM response compared to CD5- B cells, which were refractory. Therefore, the objective of this study was to further investigate the role of LCK and PD-1 signaling in AHR-mediated suppression of CD5+ ILBs. In addition, studies were conducted to establish whether IFNγ alters the levels of LCK and PD-1 in CD5+ ILBs. We found that AHR activation led to a significant upregulation of total LCK and PD-1 proteins in CD5+ ILBs, which correlated with suppression of IgM. Interestingly, treatment with recombinant IFNγ reduced LCK protein levels and reversed AHR-mediated IgM suppression in CD5+ ILBs in a similar manner as LCK inhibitors. Collectively, these results support a critical role for LCK and PD-1 in AHR-mediated suppression of the IgM response in human CD5+ ILBs.
Keywords: AhR (aryl hydrocarbon receptor); IFNgamma; IgM; Lck; PD1 (programmed cell death protein 1); TCDD; innate-like B cells.
Copyright © 2022 Zhou, Blevins, Crawford and Kaminski.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



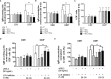



Similar articles
-
Identification of a Sensitive Human Immunological Target of Aryl Hydrocarbon Receptor Activation: CD5+ Innate-Like B Cells.Front Immunol. 2021 Apr 15;12:635748. doi: 10.3389/fimmu.2021.635748. eCollection 2021. Front Immunol. 2021. PMID: 33936048 Free PMC article.
-
Lymphocyte-Specific Protein Tyrosine Kinase (LCK) is Involved in the Aryl Hydrocarbon Receptor-Mediated Impairment of Immunoglobulin Secretion in Human Primary B Cells.Toxicol Sci. 2018 Oct 1;165(2):322-334. doi: 10.1093/toxsci/kfy133. Toxicol Sci. 2018. PMID: 29860352 Free PMC article.
-
Effects of an aryl hydrocarbon receptor ligand on human trophoblast cell development.Hum Reprod. 2025 Jun 1;40(6):1163-1172. doi: 10.1093/humrep/deaf075. Hum Reprod. 2025. PMID: 40294436
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
-
Nivolumab for adults with Hodgkin's lymphoma (a rapid review using the software RobotReviewer).Cochrane Database Syst Rev. 2018 Jul 12;7(7):CD012556. doi: 10.1002/14651858.CD012556.pub2. Cochrane Database Syst Rev. 2018. PMID: 30001476 Free PMC article.
Cited by
-
CD9 and Aryl Hydrocarbon Receptor Are Markers of Human CD19+CD14+ Atypical B Cells and Are Dysregulated in Systemic Lupus Erythematous Disease.J Immunol. 2024 Oct 15;213(8):1076-1092. doi: 10.4049/jimmunol.2400193. J Immunol. 2024. PMID: 39212542
-
B-1 cells in immunotoxicology: Mechanisms underlying their response to chemicals and particles.Front Toxicol. 2023 Apr 18;5:960861. doi: 10.3389/ftox.2023.960861. eCollection 2023. Front Toxicol. 2023. PMID: 37143777 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

