Heat Stress Weakens the Skin Barrier Function in Sturgeon by Decreasing Mucus Secretion and Disrupting the Mucosal Microbiota
- PMID: 35558118
- PMCID: PMC9087187
- DOI: 10.3389/fmicb.2022.860079
Heat Stress Weakens the Skin Barrier Function in Sturgeon by Decreasing Mucus Secretion and Disrupting the Mucosal Microbiota
Abstract
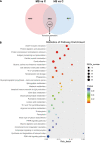
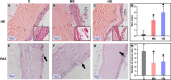
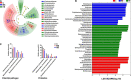
Heat stress induced by global warming has damaged the well-being of aquatic animals. The skin tissue plays a crucial role as a defense barrier to protect organism, however, little is known about the effect of heat stress on fish skin, particularly in cold-water fish species. Here, we investigated the effects of mild heat stress (24°C, MS) and high heat stress (28°C, HS) on Siberian sturgeon skin using RNA-seq, histological observation, and microbial diversity analysis. In RNA-seq, 8,819 differentially expressed genes (DEGs) in MS vs. C group and 12,814 DEGs in HS vs. C group were acquired, of which the MS vs. C and HS vs. C groups shared 3,903 DEGs, but only 1,652 DEGs were successfully annotated. The shared DEGs were significantly enriched in pathways associating with mucins synthesis. Histological observation showed that the heat stresses significantly reduced the number of skin mucous cells and induced the damages of epidermis. The microbial diversity analysis elicited that heat stress markedly disrupted the diversity and abundance of skin microbiota by increasing of potential pathogens (Vibrionimonas, Mesorhizobium, and Phyllobacterium) and decreasing of probiotics (Bradyrhizobium and Methylovirgula). In conclusion, this study reveals that heat stress causes adverse effects on sturgeon skin, reflecting in decreasing the mucus secretion and disordering the mucosal microbiota, which may contribute to develop the preventive strategy for heat stress caused by global warming.
Keywords: Siberian sturgeon; heat stress; microbial diversity; mucous cells; skin.
Copyright © 2022 Yang, Xu, Tan, Li, Li, Zhang, Feng, Chen, Jiang, Li, Du, Luo, Li, Gong, Huang, Du, Du, Liu and Wu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Effects of Citrobacter freundii on sturgeon: Insights from skin mucosal immunology and microbiota.Fish Shellfish Immunol. 2024 Jun;149:109527. doi: 10.1016/j.fsi.2024.109527. Epub 2024 Mar 30. Fish Shellfish Immunol. 2024. PMID: 38561068
-
Functional C1q is present in the skin mucus of Siberian sturgeon (Acipenser baerii).Integr Zool. 2015 Jan;10(1):102-10. doi: 10.1111/1749-4877.12100. Integr Zool. 2015. PMID: 24920077
-
Effects of Chronic Heat Stress on Kidney Damage, Apoptosis, Inflammation, and Heat Shock Proteins of Siberian Sturgeon (Acipenser baerii).Animals (Basel). 2023 Dec 2;13(23):3733. doi: 10.3390/ani13233733. Animals (Basel). 2023. PMID: 38067083 Free PMC article.
-
Heat stress on microbiota composition, barrier integrity, and nutrient transport in gut, production performance, and its amelioration in farm animals.J Anim Sci Technol. 2021 Mar;63(2):211-247. doi: 10.5187/jast.2021.e48. Epub 2021 Mar 31. J Anim Sci Technol. 2021. PMID: 33987600 Free PMC article. Review.
-
Immune relevant molecules identified in the skin mucus of fish using -omics technologies.Mol Biosyst. 2016 Jun 21;12(7):2056-63. doi: 10.1039/c5mb00890e. Mol Biosyst. 2016. PMID: 27173837 Review.
Cited by
-
Microbial Community Structure among Honey Samples of Different Pollen Origin.Antibiotics (Basel). 2023 Jan 6;12(1):101. doi: 10.3390/antibiotics12010101. Antibiotics (Basel). 2023. PMID: 36671302 Free PMC article.
-
Microbial Community Variations and Bioconversion Improvements during Soybean-Based Fermentation by Kefir Grains.Foods. 2023 Apr 8;12(8):1588. doi: 10.3390/foods12081588. Foods. 2023. PMID: 37107383 Free PMC article.
-
The Naturally Bioactive Vicine Extracted from Faba Beans Is Responsible for the Transformation of Grass Carp (Ctenopharyngodon idella) into Crisp Grass Carp.Antioxidants (Basel). 2025 Jul 1;14(7):813. doi: 10.3390/antiox14070813. Antioxidants (Basel). 2025. PMID: 40722917 Free PMC article.
-
Unveiling the Characteristics of Microbiota in Different Mucosal Layers of Leopard Coral Grouper (Plectropomus leopardus).Mar Biotechnol (NY). 2025 May 20;27(3):86. doi: 10.1007/s10126-025-10458-5. Mar Biotechnol (NY). 2025. PMID: 40392419
References
LinkOut - more resources
Full Text Sources

