Neoadjuvant chemoradiation alters the immune microenvironment in pancreatic ductal adenocarcinoma
- PMID: 35558160
- PMCID: PMC9090285
- DOI: 10.1080/2162402X.2022.2066767
Neoadjuvant chemoradiation alters the immune microenvironment in pancreatic ductal adenocarcinoma
Abstract
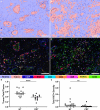
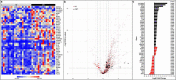
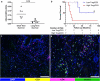
Patients with pancreatic ductal adenocarcinoma (PDAC) have a grim prognosis despite complete surgical resection and intense systemic therapies. While immunotherapies have been beneficial with many different types of solid tumors, they have almost uniformly failed in the treatment of PDAC. Understanding how therapies affect the tumor immune microenvironment (TIME) can provide insights for the development of strategies to treat PDAC. We used quantitative multiplexed immunofluorescence (qmIF) quantitative spatial analysis (qSA), and immunogenomic (IG) analysis to analyze formalin-fixed paraffin embedded (FFPE) primary tumor specimens from 44 patients with PDAC including 18 treated with neoadjuvant chemoradiation (CRT) and 26 patients receiving no treatment (NT) and compared them with tissues from 40 treatment-naïve melanoma patients. We find that relative to NT tumors, CD3+ T cell infiltration was increased in CRT treated tumors (p = .0006), including increases in CD3+CD8+ cytotoxic T cells (CTLs, p = .0079), CD3+CD4+FOXP3- T helper cells (Th, p = .0010), and CD3+CD4+FOXP3+ regulatory T cells (Tregs, p = .0089) with no difference in CD68+ macrophages. IG analysis from micro-dissected tissues indicated overexpression of genes involved in antigen presentation, T cell activation, and inflammation in CRT treated tumors. Among treated patients, a higher ratio of Tregs to total T cells was associated with shorter survival time (p = .0121). Despite comparable levels of infiltrating T cells in CRT PDACs to melanoma, PDACs displayed distinct spatial profiles with less T cell clustering as defined by nearest neighbor analysis (p < .001). These findings demonstrate that, while CRT can achieve high T cell densities in PDAC compared to melanoma, phenotype and spatial organization of T cells may limit benefit of T cell infiltration in this immunotherapy-resistant tumor.
Keywords: T regulatory cells; Tumor microenvironment; biomarkers; tumor-infiltrating lymphocytes.
© 2022 The Author(s). Published with license by Taylor & Francis Group, LLC.
Conflict of interest statement
YMS has recieved funding from Regeneron. BTF has financial interests in both Regeneron and Thermo Fisher Scientific. GAM is a consultant for CEND Biopharma and Synthekine, and has recieved funding from MERCK, Roche, BioLine, and Regeneron. None of the disclosures listed are related to this work.
Figures


References
-
- Surveillance . Epidemiology, and end results program (SEER): pancreatic cancer. 2021 ed. Bethesda (MD): National Cancer Institute (NCI; ). 2021.
-
- Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM, Faluyi O, O’Reilly DA, Cunningham D, Wadsley J, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389(10073):1011–1024. doi:10.1016/S0140-6736(16)32409-6. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
