Fabrication of a novel whole tissue-engineered intervertebral disc for intervertebral disc regeneration in the porcine lumbar spine
- PMID: 35558279
- PMCID: PMC9090940
- DOI: 10.1039/c8ra06943c
Fabrication of a novel whole tissue-engineered intervertebral disc for intervertebral disc regeneration in the porcine lumbar spine
Abstract
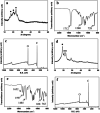
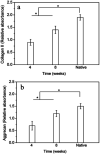
Tissue-engineered intervertebral discs (IVDs) have been proposed as a useful therapeutic strategy for the treatment of intervertebral disc degeneration (IDD). However, most studies have focused on fabrication and assessment of tissue-engineered IVDs in small animal models and the mechanical properties of the scaffolds are far below those of native human IVDs. The aim of this study was to produce a novel tissue-engineered IVD for IDD regeneration in the porcine lumbar spine. Firstly, a novel whole tissue-engineered IVD scaffold was fabricated using chitosan hydrogel to simulate the central nucleus pulposus (NP) structure, surrounded with a poly(butylene succinate-co-terephthalate) (PBST) fiber film for inner annulus fibrosus (IAF). And, a poly(ether ether ketone) (PEEK) ring was used to stimulate the outer annulus fibrosus (OAF). Then, the scaffolds were seeded with IVD cells and the cell-scaffold hybrids were transplanted into the porcine damaged spine and harvested at 4 and 8 weeks. In vitro cell experiments showed that IVD cells distributed and grew well in the scaffolds including porous hydrogel and PBST fibers. After implantation into pigs, radiographic and MRI images indicated that the tissue-engineered IVD construct could preserve the disc height in the case of discectomy as the normal disc height and maintain a large extracellular matrix and water content in the NP. Combined with the histological and gene expression results, it was concluded that the tissue-engineered IVD had similar morphological and histological structure to the natural IVD. Moreover, after implantation for 8 weeks, the tissue-engineered IVD showed a good compressive stress and elastic moduli, approaching those of natural porcine IVD. Therefore, the prepared tissue-engineered IVD construct had similar morphological and biofunctional properties to the native tissue. Also, the tissue-engineered IVD construct with excellent biocompatibility and mechanical properties provides a promising candidate for human IDD regeneration.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






Similar articles
-
The establishment and biological assessment of a whole tissue-engineered intervertebral disc with PBST fibers and a chitosan hydrogel in vitro and in vivo.J Biomed Mater Res B Appl Biomater. 2019 Oct;107(7):2305-2316. doi: 10.1002/jbm.b.34323. Epub 2019 Jan 24. J Biomed Mater Res B Appl Biomater. 2019. PMID: 30680915
-
Total disc replacement using a tissue-engineered intervertebral disc in vivo: new animal model and initial results.Evid Based Spine Care J. 2010 Aug;1(2):62-6. doi: 10.1055/s-0028-1100918. Evid Based Spine Care J. 2010. PMID: 23637671 Free PMC article.
-
High-resolution 3D printing of angle-ply annulus fibrosus scaffolds for intervertebral disc regeneration.Biofabrication. 2022 Dec 15;15(1). doi: 10.1088/1758-5090/aca71f. Biofabrication. 2022. PMID: 36541475
-
Decellularized matrix for repairing intervertebral disc degeneration: Fabrication methods, applications and animal models.Mater Today Bio. 2022 Dec 17;18:100523. doi: 10.1016/j.mtbio.2022.100523. eCollection 2023 Feb. Mater Today Bio. 2022. PMID: 36590980 Free PMC article. Review.
-
Growth and differentiation factor-5 contributes to the structural and functional maintenance of the intervertebral disc.Cell Physiol Biochem. 2015;35(1):1-16. doi: 10.1159/000369670. Epub 2015 Jan 2. Cell Physiol Biochem. 2015. PMID: 25547527 Review.
Cited by
-
Flexible support material maintains disc height and supports the formation of hydrated tissue engineered intervertebral discs in vivo.JOR Spine. 2024 Aug 5;7(3):e1363. doi: 10.1002/jsp2.1363. eCollection 2024 Sep. JOR Spine. 2024. PMID: 39104832 Free PMC article.
-
Design principles in mechanically adaptable biomaterials for repairing annulus fibrosus rupture: A review.Bioact Mater. 2023 Sep 4;31:422-439. doi: 10.1016/j.bioactmat.2023.08.012. eCollection 2024 Jan. Bioact Mater. 2023. PMID: 37692911 Free PMC article. Review.
-
Chitosan based bioactive materials in tissue engineering applications-A review.Bioact Mater. 2020 Feb 12;5(1):164-183. doi: 10.1016/j.bioactmat.2020.01.012. eCollection 2020 Mar. Bioact Mater. 2020. PMID: 32083230 Free PMC article. Review.
-
The role of biomechanical factors in models of intervertebral disc degeneration across multiple length scales.APL Bioeng. 2023 May 8;7(2):021501. doi: 10.1063/5.0137698. eCollection 2023 Jun. APL Bioeng. 2023. PMID: 37180733 Free PMC article. Review.
-
Polysaccharide-based biomaterials for regenerative therapy in intervertebral disc degeneration.Mater Today Bio. 2024 Dec 10;30:101395. doi: 10.1016/j.mtbio.2024.101395. eCollection 2025 Feb. Mater Today Bio. 2024. PMID: 39759846 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

