Alteration of glycosylation in serum proteins: a new potential indicator to distinguish non-diabetic renal diseases from diabetic nephropathy
- PMID: 35558281
- PMCID: PMC9090655
- DOI: 10.1039/c8ra06832a
Alteration of glycosylation in serum proteins: a new potential indicator to distinguish non-diabetic renal diseases from diabetic nephropathy
Abstract
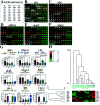
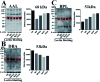
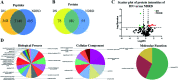
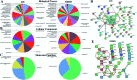
Diabetic nephropathy (DN) and nondiabetic renal disease (NDRD) are two major categories of renal diseases in diabetes mellitus patients. The clinical differentiation among them is usually not so clear and effective. In this study, sera from DN and NDRD patients were collected, and glycan profiles of serum proteins from DN and NDRD patients were investigated and compared by using lectin microarray and lectin blot. Then, altered glycoproteins were enriched by lectin coupled magnetic particle conjugate and characterized by LC-MS/MS. We found significant change in glycan patterns between DN and NDRD patients. In particular, the relative abundance of the glycopattern of Galβ1-3GalNAc which was identified by BPL (Bauhinia purpurea lectin) was significantly decreased in DN patients compared to four types of NDRD patients (p < 0.05). Moreover, BPL blotting indicated that the proteins with a molecular weight of about 53 kDa exhibited low staining signal in DN compared to all NDRD groups, which was consistent with results of lectin microarrays. After enriching by BPL and identification by LC-MS/MS, a total of 235 and 258 proteins were characterized from NDRD and DN respectively. Among these, the relative abundance of 12 isolated serum proteins exhibited significantly alteration between DN and NDRD (p < 0.05). Our findings indicated not only the relative abundance of Galβ1-3GalNAc on serum proteins but also certain glycoproteins modified with this glycopattern showed a difference between DN and NDRD patients. This suggested that the analysis of this alteration by using urine specimens may constitute an additional valuable diagnostic tool for differentiating DN and NDRD with a non-invasive method.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

