Synthesis and characterization of magnetic mesoporous Fe3O4@mSiO2-DODGA nanoparticles for adsorption of 16 rare earth elements
- PMID: 35558293
- PMCID: PMC9090902
- DOI: 10.1039/c8ra07762b
Synthesis and characterization of magnetic mesoporous Fe3O4@mSiO2-DODGA nanoparticles for adsorption of 16 rare earth elements
Abstract
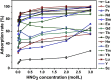
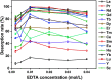
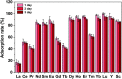
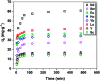
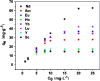
In this study, novel magnetic mesoporous Fe3O4@mSiO2-DODGA nanoparticles were prepared for efficiently adsorbing and recycling REEs. Fe3O4@mSiO2-DODGA was characterized by powder X-ray diffraction (XRD), transmission electron microscopy (TEM), vibrating sample magnetometry (VSM), Fourier transform infrared spectroscopy (FT-IR) and thermogravimetric analysis (TGA). The adsorption behavior of Fe3O4@mSiO2-DODGA was investigated by ICP-OES. The results showed that the content of DODGA in the adsorbent was 367 μmol g-1. Fe3O4@mSiO2-DODGA exhibited the highest adsorption rates for 15 REEs, except Tm, in a 2 mol L-1 nitric acid solution. Among these elements, the adsorption rates for Nd, Sm, Eu, Dy, Ho, Yb, Lu, Y and Sc ranged from 85.1% to 100.1%. The desorption rates for all 16 REE ions reached their maximum values when 0.01 mol L-1 EDTA was used as the eluent. The desorption rates for Nd, Ce, Sm, Eu, Ho, Yb, Lu, Y, and Sc were 87.7-99.8%. Fe3O4@mSiO2-DODGA had high stability in 2 mol L-1 HNO3 and could be used five times without significant loss of adsorption capacity. Moreover, these nanoparticles had high selectivity, and their adsorption rate was not affected even in a high-concentration solution of a coexisting ion. Therefore, 8 REE ions (Nd, Sm, Eu, Ho, Yb, Lu, Y, and Sc) were selected for the study of adsorption kinetics and adsorption isotherm experiments. It was demonstrated that the values of Q e (equilibrium adsorption capacity) for Nd, Sm, Eu, Ho, Yb, Lu, Y, and Sc were 14.28-60.80 mg g-1. The adsorption of REEs on Fe3O4@mSiO2-DODGA followed the pseudo-second-order kinetic model, Elovich model and Langmuir isotherm model, which indicated that the adsorption process of Fe3O4@mSiO2-DODGA for REEs comprised single-layer adsorption on a non-uniform surface controlled by chemical adsorption. It was concluded that Fe3O4@mSiO2-DODGA represents a new material for the adsorption of REEs in strongly acidic solutions.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures





References
-
- Yasuoka S. Ishida J. Kishida K. Inui H. J. Power Sources. 2017;346:56–62. doi: 10.1016/j.jpowsour.2017.02.008. - DOI
-
- Cao X. Chen L. Guo S. Fan F. Chen R. Yan A. Scr. Mater. 2017;131:24–28. doi: 10.1016/j.scriptamat.2016.12.036. - DOI
-
- Peng G. G. Zheng D. Y. Cheng C. Zhang J. Zhang H. J. Alloys Compd. 2017;693:1250–1256. doi: 10.1016/j.jallcom.2016.10.079. - DOI
-
- Hoogerstraete T. V. Binnemans K. Green Chem. 2014;16:1594–1606. doi: 10.1039/C3GC41577E. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

