Pediatric Adverse Drug Reactions: An Observational Cohort Study After Health Care Workers' Training
- PMID: 35558357
- PMCID: PMC9088431
- DOI: 10.5863/1551-6776-27.4.324
Pediatric Adverse Drug Reactions: An Observational Cohort Study After Health Care Workers' Training
Abstract
Objective: Adverse drug reactions (ADRs) in children are an important but underestimated public health issue. This study describes ADRs in a registered pediatric population of Bologna and demonstrates that ADRs might be better detected after health care personnel training.
Methods: A prospective cohort was recruited from July 1, 2016, to June 30, 2019, after health care worker sensitization, and compared to a retrospective cohort enrolled from 2013 to 2016. The ADRs are classified by system organ classes and drugs are categorized according to the Anatomical Therapeutic Chemical classification system.
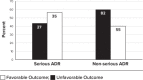
Results: We retrospectively recruited 78 pediatric patients with ADRs in the 2013 to 2016 period, and we prospectively enrolled 127 children in the 2016 to 2019 period. In both periods, most of the ADRs reported were classified as non-serious reactions (68.8%). The most frequent ADRs were general and administration site disorders. During 2013 to 2016 vaccines were the most frequent cause of ADRs (83.3%;) and the main reporters were health care workers other than physicians (84.6%), whereas during the second period, medical doctors become the main signalers (65.4%) and ADRs related to vaccines significantly decreased (55.1%). During the 2016 to 2019 period the number of drug categories was higher than in the 2013 to 2016 period (24 vs 8). Patients with ADRs due to vaccinations present more frequently a favourable outcome (63%).
Conclusions: This study demonstrates that active pharmacovigilance and health care personnel sensitization are associated with improved ADR detection, providing valuable information about drugs' safety profile in pediatric patients.
Keywords: adverse drug reactions; childhood; pharmacovigilance.
Copyright. Pediatric Pharmacy Association. All rights reserved. For permissions, email: mhelms@pediatricpharmacy.org 2022.
Conflict of interest statement
Disclosures. The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. The authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Figures
References
-
- World Health Organization The importance of pharmacovigilance. 2002. Accessed March 29, 2021. https://apps.who.int/iris/handle/10665/42493.
-
- Directive 2010/84/EU of the European Parliament and of the Council of 15 December 2010 amending, as regards pharmacovigilance, Directive 2001/83/EC on the community code relating to medicinal products for human use (text with EEA relevance) Off J Eur Union . 2010;L348:1–44. Accessed March 29, 2021. http://data.europa.eu/eli/dir/2010/84/oj/eng.
-
- Directive 2012/26/EU of the European Parliament and of the Council of 25 October 2012 amending Directive 2001/83/EC as regards pharmacovigilance (text with EEA relevance) Off J Eur Union . 2012;L299:1–4. Accessed March 29, 2021. http://data.europa.eu/eli/dir/2012/26/oj/eng.
-
- Bouquet É, Star K, Jonville-Béra AP, Durrieu G. Pharmacovigilance in pediatrics. Therapie . 2018;73(2):171–180. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials