Trophic factors intervention regenerates the nestin-expressing cell population in a model of perinatal excitotoxicity: Implications for perinatal brain injury and prematurity
- PMID: 35558521
- PMCID: PMC9094396
- DOI: 10.15761/imm.1000228
Trophic factors intervention regenerates the nestin-expressing cell population in a model of perinatal excitotoxicity: Implications for perinatal brain injury and prematurity
Abstract
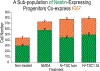
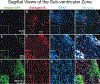
We previously showed that TSC1 (a combination of transferrin and IGF-1) is a potent inductor of myelinogenesis in myelin deficient rats and in demyelinated adult mice. More recently, we demonstrated that regeneration of oligodendrocyte progenitors and myelin are possible with a single dose of TSC1 in a mouse model of Premature birth. Here, using the same mouse model of perinatal white matter damage due to glutamate excitotoxicity (GME), we tested the hypothesis that regeneration of endogenous nestin-expressing neural progenitors improves the outcome of prematurity. Treatments: N-methyl-D-aspartate (NMDA), saline, NMDA+TSC1 together or NMDA followed byTSC1 3 days later, were stereotaxically delivered into the corpus callosum of P4 mouse pups. Fluorescence analysis showed an intense enrichment of nestin-expressing cells in groups injected with NMDA+TSC1 from which many were generated by proliferation. Moreover, when TSC1 was injected three days after the primary insult it was still able to reduce ventricular enlargement and extensively rescue nestin-expressing progenitors. Cells co-expressing the proliferation marker Ki67, CNPase and faint nestin label were more abundant in groups injected with MNDA+TSC1 at 35 days after injection. Stereological analysis showed that the number of nestin-expressing cells in the sub-ventricular zone correlated inversely with the volume of the ventricle. A delayed administration of TSC1 after excitotoxicity reduced ventriculomegaly but not as much as, when NMDA and TSC1 were injected simultaneously. Thus, the earliest TSC1 was administered, the more tissue was rescued as shown by reduced ventriculomegaly. Astrocytes responded to GME by upregulating the expression of estrogen receptor and this expression was attenuated in the presence of TSC1 suggesting a decreased inflammation and a lesser need for estrogen-mediated central nervous system (CNS) neuroprotection.
Keywords: PVL; excitotoxicity; myelination; oligodendrocyte; transferrin/IGF-1.
Conflict of interest statement
Competing interests Financial support for this work was proved in part by the National Multiple Sclerosis Society PP1498; NICHD Intellectual and Development Disabilities Research HD-04612. All other authors declare no competing interests
Figures








References
-
- Behrman RE, Butler AS (2007) “Preterm Birth Causes, Consequences, and Prevention.” The National Academies. - PubMed
-
- Holsti L, Grunau RV, Whitfield MF (2002) Developmental coordination disorder in extremely low birth weight children at nine years. J Dev Behav Pediatr 23: 9–15. - PubMed
-
- Woodward LJ, Edgin JO, Thompson D, Inder TE (2005) Object working memory deficits predicted by early brain injury and development in the preterm infant. Brain J Neurol 101: 2578–2587. - PubMed
-
- Volpe JJ (2005) Encephalopathy of Prematurity Includes Neuronal Abnormalities. Pediatrics 116: 221–225. - PubMed
-
- Lindquist S (1986) The heat-shock response. Annu Rev Biochem 55: 1151–1191. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
