Fabrication of cotton textile waste-based magnetic activated carbon using FeCl3 activation by the Box-Behnken design: optimization and characteristics
- PMID: 35558585
- PMCID: PMC9089844
- DOI: 10.1039/c8ra06253f
Fabrication of cotton textile waste-based magnetic activated carbon using FeCl3 activation by the Box-Behnken design: optimization and characteristics
Abstract
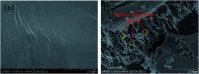
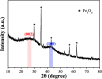
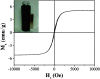
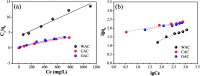
Cotton textile waste-based magnetic activated carbon was prepared via simultaneous activation-pyrolysis using FeCl3 as a novel activating agent. The response surface methodology based on the Box-Behnken design method was applied to optimize the preparation parameters and predict the specific surface area of the samples. The optimal activated carbon was obtained at a mass ratio of FeCl3/CTW, activation time and activation temperature of 1.62 : 1, 1 h and 700 °C, respectively. The experimental maximum yield and iodine adsorptive value (32.66% and 714.55 mg g-1) of the resultant carbon were close to that of the predicated response values (34.85% and 783.75 mg g-1), respectively. SEM, N2 adsorption-desorption isotherms, XRD, PPMS, FTIR and pHpzc measurements were conducted to analyze the physicochemical characteristics of the optimal sample. The results showed that the carbon matrix had a high specific surface area of 837.39 m2 g-1 with abundant micropores and acidic surface functional groups, and the saturation magnetization (Ms) was 5.2 emu g-1 due to the formation of Fe3O4. The maximum adsorption of Cr(vi) by the carbon reached 212.77 mg g-1. Furthermore, the addition of FeCl3 lowered the pyrolytic carbonization temperature and inhibited the generation of volatiles in the activation-pyrolysis process. Meanwhile, the formation of Fe2O3 and Fe3O4 derived from FeCl3 was beneficial for the development of vast micropores.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




Similar articles
-
Conversion of cotton textile wastes into porous carbons by chemical activation with ZnCl2, H3PO4, and FeCl3.Environ Sci Pollut Res Int. 2020 Jul;27(20):25186-25196. doi: 10.1007/s11356-020-08873-3. Epub 2020 Apr 28. Environ Sci Pollut Res Int. 2020. PMID: 32342420
-
Synthesis of char-based adsorbents from cotton textile waste assisted by iron salts at low pyrolysis temperature for Cr(VI) removal.Environ Sci Pollut Res Int. 2020 Apr;27(10):11012-11025. doi: 10.1007/s11356-019-07588-4. Epub 2020 Jan 17. Environ Sci Pollut Res Int. 2020. PMID: 31953756
-
Understanding reactions and pore-forming mechanisms between waste cotton woven and FeCl3 during the synthesis of magnetic activated carbon.Chemosphere. 2020 Feb;241:125120. doi: 10.1016/j.chemosphere.2019.125120. Epub 2019 Oct 14. Chemosphere. 2020. PMID: 31683447
-
Facile preparation of magnetic porous carbon monolith from waste corrugated cardboard box for solar steam generation and adsorption.Biomass Convers Biorefin. 2022;12(6):2185-2202. doi: 10.1007/s13399-020-00739-5. Epub 2020 May 8. Biomass Convers Biorefin. 2022. PMID: 32395400 Free PMC article.
-
CO2 capture by adsorption on biomass-derived activated char: A review.Sci Total Environ. 2021 Dec 1;798:149296. doi: 10.1016/j.scitotenv.2021.149296. Epub 2021 Jul 26. Sci Total Environ. 2021. PMID: 34325142 Review.
Cited by
-
Possibility Routes for Textile Recycling Technology.Polymers (Basel). 2021 Nov 6;13(21):3834. doi: 10.3390/polym13213834. Polymers (Basel). 2021. PMID: 34771390 Free PMC article. Review.
-
A Facile Approach to Produce Activated Carbon from Waste Textiles via Self-Purging Microwave Pyrolysis and FeCl3 Activation for Electromagnetic Shielding Applications.Polymers (Basel). 2024 Mar 26;16(7):915. doi: 10.3390/polym16070915. Polymers (Basel). 2024. PMID: 38611173 Free PMC article.
References
-
- Nahil M. A. Williams P. T. J. Anal. Appl. Pyrolysis. 2010;89:51–59. doi: 10.1016/j.jaap.2010.05.005. - DOI
-
- National and Reform Commission, Recyclable Resources and Circular Economy, 2014, vol. 7, pp. 2–6
-
- Zamani B., Carbon footprint and energy use of textile recycling techniques, Chalmers University of Technology, 2011
-
- Wang Y. J. Waste Biomass Valorization. 2010;1:135–143. doi: 10.1007/s12649-009-9005-y. - DOI
-
- Wang S. H. Wei M. X. Xu Q. L. Jia H. S. Fibers Polym. 2016;17:212–219. doi: 10.1007/s12221-016-5749-8. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

