K6P2W18O62 encapsulated into magnetic Fe3O4/MIL-101 (Cr) metal-organic framework: a novel magnetically recoverable nanoporous adsorbent for ultrafast treatment of aqueous organic pollutants solutions
- PMID: 35558601
- PMCID: PMC9089921
- DOI: 10.1039/c8ra06287k
K6P2W18O62 encapsulated into magnetic Fe3O4/MIL-101 (Cr) metal-organic framework: a novel magnetically recoverable nanoporous adsorbent for ultrafast treatment of aqueous organic pollutants solutions
Abstract
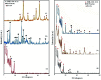
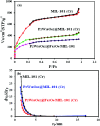
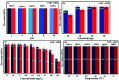
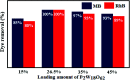
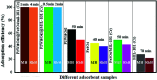
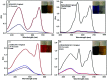
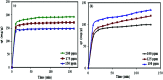
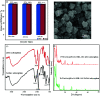
In this study, a Wells-Dawson type K6P2W18O62 polyoxometalate was encapsulated into the magnetic Fe3O4/MIL-101 (Cr) metal-organic framework and applied as a new magnetically recoverable ternary adsorbent to remove organic dyes from aqueous solutions. The as-prepared ternary magnetically recyclable hybrid (denoted as P2W18O62@Fe3O4/MIL-101 (Cr)) was characterized by FT-IR spectroscopy, powder X-ray diffraction (XRD), Raman spectroscopy, EDX, SEM, BET surface area, and magnetic measurements. The results showed the successful encapsulation of K6P2W18O62 (∼26.5 wt%) into the magnetic Fe3O4/MIL-101 (Cr) framework. The magnetic hybrid had a high specific surface area of 934.89 m2 g-1. The adsorption efficiency of this nanohybrid for the removal of methylene blue (MB), rhodamine B (RhB), and methyl orange (MO) from aqueous solutions was evaluated. The magnetic nanohybrid demonstrated the fast and selective adsorption of cationic dyes from mixed dye solutions. The adsorption rate and capacity of P2W18O62@Fe3O4/MIL-101 (Cr) were increased as compared with MIL-101 (Cr), P2W18O62, and Fe3O4/MIL-101 samples due to the increased electrostatic attraction. The effects of parameters such as the adsorbent dosage, temperature, dye concentration, and pH were investigated on the adsorption process. The adsorption kinetics was analyzed by the Freundlich, Langmuir, and Temkin isotherm models and pseudo-second-order and pseudo-first-order kinetics models, with the Langmuir isotherm and pseudo-second-order kinetic model found to be suitable to describe the equilibrium data. Also, the thermodynamic results of the nanohybrid indicated that the adsorption was an endothermic and spontaneous process. After the adsorption reaction, the magnetic nanohybrid could be easily separated and reused without any change in structure. Based on the results of this study, the nanohybrid was an efficient adsorbent for eliminating cationic dyes.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures







References
LinkOut - more resources
Full Text Sources
Miscellaneous

