Bicyclic 6 + 6 systems: the chemistry of pyrimido[4,5- d]pyrimidines and pyrimido[5,4- d]pyrimidines
- PMID: 35558733
- PMCID: PMC9092567
- DOI: 10.1039/c9ra05687d
Bicyclic 6 + 6 systems: the chemistry of pyrimido[4,5- d]pyrimidines and pyrimido[5,4- d]pyrimidines
Abstract
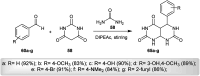
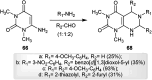
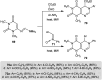
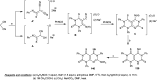
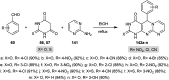
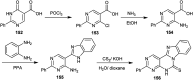
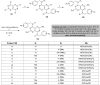
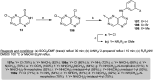
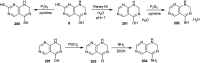
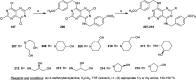
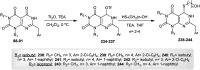
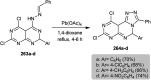
The present study provides an overview of the chemistry and biological significance of pyrimido[4,5-d]pyrimidine and pyrimido[5,4-d]pyrimidine analogs as types of bicyclic [6 + 6] systems. The main sections include: (1) synthesis methods; (2) the reactivities of the substituents linked to the ring carbon and nitrogen atoms; and (3) biological applications. A discussion demonstrating the proposed mechanisms of unexpected synthetic routes is intended. The aim of this study is to discuss the synthetic significance of the titled compounds and to establish the biological characteristics of this class of compounds as studied to date, where the compounds have been applied on a large scale in the medical and pharmaceutical fields. This survey will help researchers in the fields of synthetic organic and medicinal chemistry to undertake and improve new approaches for the construction of new standard biological components.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







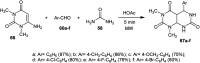






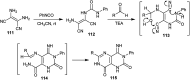

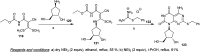
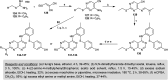
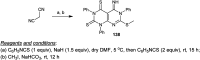




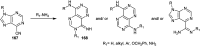










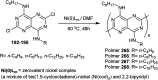






Similar articles
-
Advances in the chemical and biological diversity of heterocyclic systems incorporating pyrimido[1,6-a]pyrimidine and pyrimido[1,6-c]pyrimidine scaffolds.RSC Adv. 2020 Apr 20;10(26):15461-15492. doi: 10.1039/d0ra00411a. eCollection 2020 Apr 16. RSC Adv. 2020. PMID: 35558641 Free PMC article. Review.
-
Advances in the Chemistry of 6-6 Bicyclic Systems: Chemistry of Pyrido[3,4- d]pyrimidines.Curr Org Synth. 2019;16(6):812-854. doi: 10.2174/1570179416666190704113647. Curr Org Synth. 2019. PMID: 31984909
-
Bicyclic 5-6 Systems: Comprehensive Synthetic Strategies for the Annulations of Pyrazolo[ 1,5-a]pyrimidines.Curr Org Synth. 2021 Oct 26;18(6):547-586. doi: 10.2174/1570179418666210509015108. Curr Org Synth. 2021. PMID: 33966620 Review.
-
Antitumor and antiviral activity of synthetic alpha- and beta-ribonucleosides of certain substituted pyrimido[5,4-d]pyrimidines: a new synthetic strategy for exocyclic aminonucleosides.J Med Chem. 1989 Mar;32(3):629-37. doi: 10.1021/jm00123a022. J Med Chem. 1989. PMID: 2918511
-
Pyrido[2, 3-d]pyrimidines and pyrimido[5',4':5, 6]pyrido[2, 3-d]pyrimidines as new antiviral agents: synthesis and biological activity.Arch Pharm (Weinheim). 2002 Jun;335(6):289-95. doi: 10.1002/1521-4184(200208)335:6<289::AID-ARDP289>3.0.CO;2-Z. Arch Pharm (Weinheim). 2002. PMID: 12210772
Cited by
-
Updates on Intrinsic Medicinal Chemistry of 1,4-dihydropyridines, Perspectives on Synthesis and Pharmacokinetics of Novel 1,4-dihydropyrimidines as Calcium Channel Blockers: Clinical Pharmacology.Curr Top Med Chem. 2025;25(11):1351-1376. doi: 10.2174/0115680266323908241114064318. Curr Top Med Chem. 2025. PMID: 39754778 Review.
-
Recent advancements in the multicomponent synthesis of heterocycles integrated with a pyrano[2,3-d]pyrimidine core.RSC Adv. 2022 Apr 19;12(19):11808-11842. doi: 10.1039/d2ra00927g. eCollection 2022 Apr 13. RSC Adv. 2022. PMID: 35481073 Free PMC article. Review.
-
Insights into the recent progress in the medicinal chemistry of pyranopyrimidine analogs.RSC Med Chem. 2022 May 6;13(5):522-567. doi: 10.1039/d2md00076h. eCollection 2022 May 25. RSC Med Chem. 2022. PMID: 35694689 Free PMC article. Review.
-
Synthetic strategies of heterocycle-integrated pyridopyrimidine scaffolds supported by nano-catalysts.RSC Adv. 2023 Apr 14;13(17):11600-11634. doi: 10.1039/d3ra00922j. eCollection 2023 Apr 11. RSC Adv. 2023. PMID: 37063723 Free PMC article. Review.
-
Advances in the chemical and biological diversity of heterocyclic systems incorporating pyrimido[1,6-a]pyrimidine and pyrimido[1,6-c]pyrimidine scaffolds.RSC Adv. 2020 Apr 20;10(26):15461-15492. doi: 10.1039/d0ra00411a. eCollection 2020 Apr 16. RSC Adv. 2020. PMID: 35558641 Free PMC article. Review.
References
-
- Gastpar H. Acta Med. Scand., Suppl. 1972;525:269–271. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

