Effects of Buffalo Milk and Cow Milk on Lipid Metabolism in Obese Mice Induced by High Fat
- PMID: 35558744
- PMCID: PMC9089190
- DOI: 10.3389/fnut.2022.841800
Effects of Buffalo Milk and Cow Milk on Lipid Metabolism in Obese Mice Induced by High Fat
Abstract
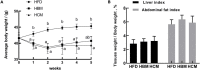
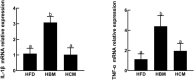
The aim of this study was to evaluate the effects of buffalo milk and cow milk on lipid metabolism in obese mice. Milk composition analysis showed fat, protein, and total solid content in buffalo milk was higher than cow milk, while the lactose content of buffalo milk was lower than cow milk. After milk metabolite extraction and LC-MS/MS analysis, differential metabolites were mainly enriched in "linoleic acid metabolism pathways," "pentose and glucuronate interconversion pathways," and "metabolism of xenobiotics by cytochrome P450 pathways." We fed three groups of C57BL/6J mice (n = 6 per group) for 5 weeks: (1) high-fat diet group (HFD group); (2) high-fat diet + buffalo milk group (HBM group); and (3) high-fat diet + cow milk group (HCM group). Our results showed that body weight of mice was significantly decreased in HBM and HCM groups from 1 to 4 weeks compared with the HFD group. The mRNA expression of ACAA2, ACACB, and SLC27A5 genes involved in the lipid metabolism in liver tissue were significantly elevated in HCM group, relatively to HFD and HBM group. In addition, the adipocyte number, size and lipid accumulation in the liver were significantly decreased in HCM group compared with the HFD group by H&E staining and oil red O staining, but was not change in HBM group. The mRNA levels of TNF-α and IL-1β inflammatory genes were significantly increased in HBM group, relatively to HFD and HCM group, which is consistent with results from inflammatory cell infiltration and tissue disruption by colon tissue sections. In conclusion, dietary supplementation of cow milk has beneficial effects on loss of weight and lipid metabolism in obese mice.
Keywords: buffalo; inflammatory; lipid metabolism; metabonomic; milk.
Copyright © 2022 Jiang, Meng, Cheng, Zhan, Ma, Yang, Huang, Yan, Gong and Zhao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous

