Microwave irradiation: a green approach for the synthesis of functionalized N-methyl-1,4-dihydropyridines
- PMID: 35558769
- PMCID: PMC9091863
- DOI: 10.1039/c8ra09155b
Microwave irradiation: a green approach for the synthesis of functionalized N-methyl-1,4-dihydropyridines
Abstract
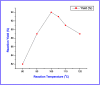
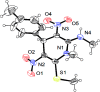
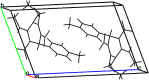
An eco-friendly and cost-effective, microwave-assisted green approach has been developed for the synthesis of diverse functionalized N-methyl-1,4-dihydropyridines (1,4-DHPs). This pseudo three-component reaction was carried out between two equivalents of (E)-N-methyl-1-(methylthio)-2-nitroethenamine (NMSM) and one equivalent of aromatic aldehydes under microwave irradiation at 100 °C without catalyst and solvent. Short reaction times, avoidance of toxic solvents or expensive, metallic and corrosive catalysts and no need for column chromatographic purification are among the valuable features of the presented method. Moreover, the "greenness" of the method was evaluated within the ambits of the defined green metrics such as atom economy, carbon efficiency, E-factor, reaction mass efficiency, overall efficiency, process mass intensity and solvent intensity and the method exhibited a good to excellent score.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

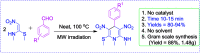

References
-
- Sheldon R. A. Chem. Soc. Rev. 2012;41:1437–1451. doi: 10.1039/C1CS15219J. - DOI - PubMed
- Gupta P. Paul S. Catal. Today. 2014;236:153–170. doi: 10.1016/j.cattod.2014.04.010. - DOI
- Cioc R. C. Ruijter E. Orru R. V. A. Green Chem. 2014;16:2958–2975. doi: 10.1039/C4GC00013G. - DOI
- de Graaff C. Ruijter E. Orru R. V. A. Chem. Soc. Rev. 2012;41:3969–4009. doi: 10.1039/C2CS15361K. - DOI - PubMed
-
- Alvim H. G. O. Pinheiro D. L. J. Carvalho-Silva V. H. Fioramonte M. Gozzo F. C. da Silva W. A. Amarante G. W. Neto B. A. D. J. Org. Chem. 2018;83:12143–12153. doi: 10.1021/acs.joc.8b02101. - DOI - PubMed
- Zeng L. Huang B. Shen Y. Cui S. Org. Lett. 2018;20:3460–3464. doi: 10.1021/acs.orglett.8b01159. - DOI - PubMed
- Rotstein B. H. Zaretsky S. Rai V. Yudin A. K. Chem. Rev. 2014;114:8323–8359. doi: 10.1021/cr400615v. - DOI - PubMed
- Zhu J. and Bienayme H., Multicomponent reactions, Wiley-VCH, Weinheim, Germany, 1st edn, 2005
-
- Alvim H. G. O. Correa J. R. Assumpcao J. A. F. da Silva W. A. Rodrigues M. O. de Macedo J. L. Fioramonte M. Gozzo F. C. Gatto C. C. Neto B. A. D. J. Org. Chem. 2018;83:4044–4053. doi: 10.1021/acs.joc.8b00472. - DOI - PubMed
- Toure B. B. Hall D. G. Chem. Rev. 2009;109:4439–4486. doi: 10.1021/cr800296p. - DOI - PubMed
- Ramon D. J. Yus M. Angew. Chem., Int. Ed. 2005;44:1602–1634. doi: 10.1002/anie.200460548. - DOI - PubMed
- Wan J.-P. Liu Y. Curr. Org. Chem. 2011;15:2758–2773. doi: 10.2174/138527211796378479. - DOI
-
- Bagdi A. K. Santra S. Monir K. Hajra A. Chem. Commun. 2015;51:1555–1575. doi: 10.1039/C4CC08495K. - DOI - PubMed
- Boukis A. C. Reiter K. Frolich M. Hofheinz D. Meier M. A. R. Nat. Commun. 2018;9:1439–1449. doi: 10.1038/s41467-018-03784-x. - DOI - PMC - PubMed
- Parikh N. Roy S. R. Seth K. Kumar A. Chakraborti A. K. Synthesis. 2016;48:547–556.
-
- Khan M. M. Yousuf R. Khan S. Shafiullah RSC Adv. 2015;5:57883–57905. doi: 10.1039/C5RA08059B. - DOI
- Titze L. F. Modi A. Med. Res. Rev. 2000;20:304–322. doi: 10.1002/1098-1128(200007)20:4<304::AID-MED3>3.0.CO;2-8. - DOI - PubMed
- Ugi I. Dömling A. Werner B. J. Heterocycl. Chem. 2000;37:647–658. doi: 10.1002/jhet.5570370322. - DOI
- Tempest P. A. Curr. Opin. Drug Discovery Dev. 2005;8:776–788. - PubMed
- Bienayme H. Hulme C. Oddon G. Schmitt P. Chem.–Eur. J. 2000;6:3321–3329. doi: 10.1002/1521-3765(20000915)6:18<3321::AID-CHEM3321>3.0.CO;2-A. - DOI - PubMed
LinkOut - more resources
Full Text Sources

