Enantioselective Michael addition of malonates to α,β-unsaturated ketones catalyzed by 1,2-diphenylethanediamine
- PMID: 35558786
- PMCID: PMC9091971
- DOI: 10.1039/c8ra07809b
Enantioselective Michael addition of malonates to α,β-unsaturated ketones catalyzed by 1,2-diphenylethanediamine
Abstract
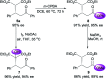
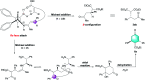
A general and highly enantioselective Michael addition of malonates to cinnamones and chalcones has been developed. The commercially available 1,2-diphenylethanediamine could be directly utilized as the organocatalyst to furnish the desired adducts in satisfactory yield (61-99%) and moderate to excellent enantiopurity (65 to >99% ee). β-Ketoester was also a competent donor and was employed to construct densely functionalized cyclohexenones via a tandem Michael-aldol condensation process.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
References
-
- Sibi M. P. Manyem S. Tetrahedron. 2000;56:8033. doi: 10.1016/S0040-4020(00)00618-9. - DOI
- Krause N. Hoffmann-Röder A. Synthesis. 2001;2001:0171. doi: 10.1055/s-2001-10803. - DOI
- Christoffers J. Koripelly G. Rosiak A. Rössle M. Synthesis. 2007;2007:1279. doi: 10.1055/s-2007-966005. - DOI
- Tsogoeva S. B. Eur. J. Org. Chem. 2007;2007:1701. doi: 10.1002/ejoc.200600653. - DOI
- Almaşi D. Alonso D. A. Nájera C. Tetrahedron: Asymmetry. 2007;18:299. doi: 10.1016/j.tetasy.2007.01.023. - DOI
-
- Ooi T. Ohara D. Fukumoto K. Maruoka K. Org. Lett. 2005;7:3195. doi: 10.1021/ol050902a. - DOI - PubMed
- Kottala Vijaya P. Murugesan S. Siva A. Tetrahedron Lett. 2015;56:5209. doi: 10.1016/j.tetlet.2015.07.074. - DOI
- Kim D. Y. Huh S. C. Kim S. M. Tetrahedron Lett. 2001;42:6299. doi: 10.1016/S0040-4039(01)01237-0. - DOI
- Dere R. T. Pal R. R. Patil P. S. Salunkhe M. M. Tetrahedron Lett. 2003;44:5351. doi: 10.1016/S0040-4039(03)01198-5. - DOI
LinkOut - more resources
Full Text Sources