Facile fabrication of multifunctional fabrics: use of copper and silver nanoparticles for antibacterial, superhydrophobic, conductive fabrics
- PMID: 35558807
- PMCID: PMC9091953
- DOI: 10.1039/c8ra08310j
Facile fabrication of multifunctional fabrics: use of copper and silver nanoparticles for antibacterial, superhydrophobic, conductive fabrics
Abstract
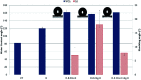
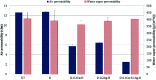
This study aims to develop a multifunctional fabric for antibacterial, superhydrophobic and conductive performance using a facile fabrication method. Conductive metal particles, copper and silver, were used as antibacterial agents as well as a means to create nanoscale roughness on the fabric surface. Subsequent hydrophobic coating with 1-dodecanethiol produced a superhydrophobic surface. The single metal treatment with Cu or Ag, and the combined metal treatment of Cu/Ag were compared for the multifunctionality. The Cu/Ag treated fabric and the Cu treated fabric showed a bacteriostatic rate ≥ 99% and a sterilization rate ≥ 99% against S. aureus, suggesting a higher antibacterial activity against the Gram-positive bacteria. In contrast, the Ag treated fabric showed a lower antibacterial effect regardless of the bacteria type. With regards to conductivity, the single metal treated fabric did not exhibit conductivity; however the Cu/Ag treated fabric showed a high level conductivity with a surface resistivity of 25.17 ± 8.18 Ω sq-1 and 184.38 ± 85.42 Ω sq-1 before and after hydrophobic coating, respectively. Fabrics treated with Cu and Cu/Ag particles (with hydrophobic coating) displayed superhydrophobic characteristics with the contact angle of 161-162° and the shedding angle of 7.0-7.8°. The air permeability decreased after the particle treatment as the particles blocked the pores in the fabric. However, the water vapor permeability and tensile strength were not significantly affected by the particle treatment. This study is significant in that a multifunctionality of antibacterial effect, superhydrophobicity, and conductivity was achieved through the facile processes for metal nanoparticle attachment and hydrophobic coating. The multifunctional fabrics produced in this study can be practically applied to self-cleaning smart clothing, which has reduced laundering need, without hygiene concerns.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures







Similar articles
-
Superhydrophobicity and conductivity of polyester-conductive fabrics using alkaline hydrolysis.RSC Adv. 2022 Aug 15;12(35):22911-22921. doi: 10.1039/d2ra03996f. eCollection 2022 Aug 10. RSC Adv. 2022. PMID: 36106007 Free PMC article.
-
Antibacterial Superhydrophobic Cotton Fabric with Photothermal, Self-Cleaning, and Ultraviolet Protection Functionalities.ACS Appl Mater Interfaces. 2023 Jul 19;15(28):34031-34043. doi: 10.1021/acsami.3c04598. Epub 2023 Jul 3. ACS Appl Mater Interfaces. 2023. PMID: 37399520
-
Multifunctional cotton non-woven fabrics coated with silver nanoparticles and polymers for antibacterial, superhydrophobic and high performance microwave shielding.J Colloid Interface Sci. 2021 Jan 15;582(Pt A):112-123. doi: 10.1016/j.jcis.2020.08.037. Epub 2020 Aug 11. J Colloid Interface Sci. 2021. PMID: 32814219
-
Electric heated cotton fabrics with durable conductivity and self-cleaning properties.RSC Adv. 2018 Sep 4;8(54):31008-31018. doi: 10.1039/c8ra05530k. eCollection 2018 Aug 30. RSC Adv. 2018. PMID: 35548731 Free PMC article.
-
Recent Advances in Superhydrophobic and Antibacterial Cellulose-Based Fibers and Fabrics: Bio-inspiration, Strategies, and Applications.Adv Fiber Mater. 2023 May 22:1-37. doi: 10.1007/s42765-023-00297-1. Online ahead of print. Adv Fiber Mater. 2023. PMID: 37361104 Free PMC article. Review.
Cited by
-
Interaction of Surface Energy Components between Solid and Liquid on Wettability, and Its Application to Textile Anti-Wetting Finish.Polymers (Basel). 2019 Mar 14;11(3):498. doi: 10.3390/polym11030498. Polymers (Basel). 2019. PMID: 30960482 Free PMC article.
-
Antibacterial and Antiviral Functional Materials: Chemistry and Biological Activity toward Tackling COVID-19-like Pandemics.ACS Pharmacol Transl Sci. 2020 Dec 29;4(1):8-54. doi: 10.1021/acsptsci.0c00174. eCollection 2021 Feb 12. ACS Pharmacol Transl Sci. 2020. PMID: 33615160 Free PMC article. Review.
-
Superhydrophobicity and conductivity of polyester-conductive fabrics using alkaline hydrolysis.RSC Adv. 2022 Aug 15;12(35):22911-22921. doi: 10.1039/d2ra03996f. eCollection 2022 Aug 10. RSC Adv. 2022. PMID: 36106007 Free PMC article.
-
Quantification Methods for Textile-Adhered Bacteria: Extraction, Colorimetric, and Microscopic Analysis.Polymers (Basel). 2019 Oct 12;11(10):1666. doi: 10.3390/polym11101666. Polymers (Basel). 2019. PMID: 31614838 Free PMC article.
-
Antimicrobial Properties of the Ag, Cu Nanoparticle System.Biology (Basel). 2021 Feb 10;10(2):137. doi: 10.3390/biology10020137. Biology (Basel). 2021. PMID: 33578705 Free PMC article. Review.
References
-
- Son E. J. Son S. G. Hwang Y. G. Fiber Technol. Ind. 2012;16:65–76.
-
- Fu Y. Jiang J. Zhang Q. Zhan X. Chen F. J. Mater. Chem. A. 2016;5:275–284. doi: 10.1039/C6TA06481G. - DOI
-
- Wang H. Wang Z.-M. Yan X. Chen J. Lang W.-Z. Guo Y.-J. J. Ind. Eng. Chem. 2017;52:295–304. doi: 10.1016/j.jiec.2017.03.059. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

