pH-Induced Changes in the SERS Spectrum of Thiophenol at Gold Electrodes during Cyclic Voltammetry
- PMID: 35558822
- PMCID: PMC9082592
- DOI: 10.1021/acs.jpcc.2c00416
pH-Induced Changes in the SERS Spectrum of Thiophenol at Gold Electrodes during Cyclic Voltammetry
Abstract
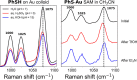
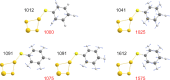
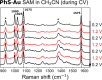
Thiophenol is a model compound used in the study of self-assembly of arylthiols on gold surfaces. In particular, changes in the surface-enhanced Raman scattering (SERS) spectra of these self-assembled monolayers (SAMs) with a change of conditions have been ascribed to, for example, differences in orientation with respect to the surface, protonation state, and electrode potential. Here, we show that potential-induced changes in the SERS spectra of SAMs of thiophenol on electrochemically roughened gold surfaces can be due to local pH changes at the electrode. The changes observed during the potential step and cyclic voltammetry experiments are identical to those induced by acid-base switching experiments in a protic solvent. The data indicate that the potential-dependent spectral changes, assigned earlier to changes in molecular orientation with respect to the surface, can be ascribed to changes in the pH locally at the electrode. The pH at the electrode can change as much as several pH units during electrochemical measurements that reach positive potentials where oxidation of adventitious water can occur. Furthermore, once perturbed by applying positive potentials, the pH at the electrode takes considerable time to recover to that of the bulk solution. It is noted that the changes in pH even during cyclic voltammetry in organic solvents can be equivalent to the addition of strong acids, such as CF3SO3H, and such effects should be considered in the study of the redox chemistry of pH-sensitive redox systems and potential-dependent SERS in particular.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Characterization of a Self-Assembled Monolayer of 1-Thio-β-D-Glucose with Electrochemical Surface Enhanced Raman Spectroscopy Using a Nanoparticle Modified Gold Electrode.Langmuir. 2015 Sep 15;31(36):10076-86. doi: 10.1021/acs.langmuir.5b02767. Epub 2015 Sep 2. Langmuir. 2015. PMID: 26313341
-
Surface-enhanced Raman scattering studies of 1,10-phenanthroline adsorption and its surface complexes on a gold electrode.J Phys Chem B. 2005 Jun 2;109(21):10880-5. doi: 10.1021/jp0443198. J Phys Chem B. 2005. PMID: 16852324
-
Neuromedin C: potential-dependent surface-enhanced Raman spectra in the far-red spectral region on silver, gold, and copper surfaces.J Phys Chem B. 2010 Apr 22;114(15):5117-24. doi: 10.1021/jp910575f. J Phys Chem B. 2010. PMID: 20349931
-
Raman mapping and in situ SERS spectroelectrochemical studies of 6-mercaptopurine SAMs on the gold electrode.J Phys Chem B. 2005 Feb 24;109(7):2739-44. doi: 10.1021/jp046082l. J Phys Chem B. 2005. PMID: 16851282
-
[New progress of surface enhanced Raman spectroscopic studies on a gold electrode in a nonaqueous system].Guang Pu Xue Yu Guang Pu Fen Xi. 2001 Jun;21(3):308-10. Guang Pu Xue Yu Guang Pu Fen Xi. 2001. PMID: 12947653 Review. Chinese.
Cited by
-
Selective Analysis of Redox Processes at the Electrode Interface with Time-Resolved Raman Spectroscopy.Langmuir. 2023 Aug 1;39(30):10383-10394. doi: 10.1021/acs.langmuir.3c00633. Epub 2023 Jul 21. Langmuir. 2023. PMID: 37477006 Free PMC article.
-
Exploring Surface-Enhanced Raman Spectroscopy of Pyrazine-2-Carbonitrile for Indirect Label-Free Albumin Quantification in an In Vitro Endothelium Permeability Assay.Anal Chem. 2025 Feb 25;97(7):4075-4083. doi: 10.1021/acs.analchem.4c05906. Epub 2025 Feb 10. Anal Chem. 2025. PMID: 39928859 Free PMC article.
-
Seeing an Unobservable Fe(III)/Fe(IV) Redox Process of the Nonheme Iron N4Py Complex by High-Speed Surface-Enhanced Raman Spectroelectrochemistry.Inorg Chem. 2025 Jun 2;64(21):10549-10557. doi: 10.1021/acs.inorgchem.5c01017. Epub 2025 May 20. Inorg Chem. 2025. PMID: 40391616 Free PMC article.
-
Investigating Perampanel Antiepileptic Drug by DFT Calculations and SERS with Custom Spinning Cell.Molecules. 2023 Aug 9;28(16):5968. doi: 10.3390/molecules28165968. Molecules. 2023. PMID: 37630222 Free PMC article.
-
Application of a Strong Base Anion Exchange Resin for the Removal of Thiophenol from Aqueous Solution.Molecules. 2025 Jan 24;30(3):525. doi: 10.3390/molecules30030525. Molecules. 2025. PMID: 39942629 Free PMC article.
References
-
- Nuzzo R. G.; Allara D. L. Adsorption of Bifunctional Organic Disulfides on Gold Surfaces. J. Am. Chem. Soc. 1983, 105, 4481–4483. 10.1021/ja00351a063. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
