Structure-activity correlation of thermally activated graphite electrodes for vanadium flow batteries
- PMID: 35558842
- PMCID: PMC9092384
- DOI: 10.1039/d2ra02368g
Structure-activity correlation of thermally activated graphite electrodes for vanadium flow batteries
Abstract
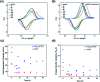
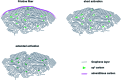
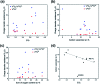
Thermal activation of graphite felts has proven to be a valuable technique for electrodes in vanadium flow batteries to improve their sluggish reaction kinetics. In the underlying work, a novel approach is presented to describe the morphological, microstructural, and chemical changes that occur as a result of the activation process. All surface properties were monitored at different stages of thermal activation and correlated with the electrocatalytic activity. The subsequently developed model consists of a combined ablation and damaging process observed by Raman spectroscopy, X-ray photoelectron spectroscopy and scanning electron microscopy. Initially, the outermost layer of adventitious carbon is removed and sp2 layers of graphite are damaged in the oxidative atmosphere, which enhances the electrocatalytic activity by introducing small pores with sharp edges. In later stages, the concentration of reaction sites does not increase further, but the defect geometry changes significantly, leading to lower activity. This new perspective on thermal activation allows several correlations between structural and functional properties of graphite for the vanadium redox couple, describing the importance of structural defects over surface chemistry.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
-
- Da Silva Lima L. Quartier M. Buchmayr A. Sanjuan-Delmás D. Laget H. Corbisier D. Mertens J. Dewulf J. Life cycle assessment of lithium-ion batteries and vanadium redox flow batteries-based renewable energy storage systems. Sustain. Energy Technol. Assess. 2021;46:101286.
-
- Lv Y. Han C. Zhu Y. Zhang T. Yao S. He Z. Dai L. Wang L. Recent advances in metals and metal oxides as catalysts for vanadium redox flow battery: properties, structures, and perspectives. J. Mater. Sci. Technol. 2021;75:96. doi: 10.1016/j.jmst.2020.09.042. - DOI
-
- Schnucklake M. Cheng M. Maleki M. Roth C. A mini-review on decorating, templating of commercial and electrospinning of new porous carbon electrodes for vanadium redox flow batteries. JPhys Mater. 2021;4:32007. doi: 10.1088/2515-7639/abf1a9. - DOI
-
- Taş M. Elden G. A comprehensive review of carbon‐based and metal‐based electrocatalysts in the vanadium redox flow battery. Energy Storage. 2022;4:e265. doi: 10.1002/est2.265. - DOI
LinkOut - more resources
Full Text Sources

