Ultrasound-assisted transition-metal-free catalysis: a sustainable route towards the synthesis of bioactive heterocycles
- PMID: 35558846
- PMCID: PMC9092113
- DOI: 10.1039/d2ra02063g
Ultrasound-assisted transition-metal-free catalysis: a sustainable route towards the synthesis of bioactive heterocycles
Abstract
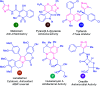
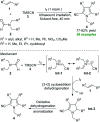
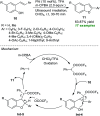
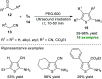
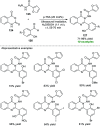
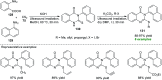
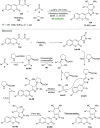
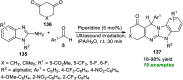
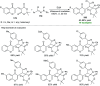
Heterocycles of synthetic and natural origin are a well-established class of compounds representing a broad range of organic molecules that constitute over 60% of drugs and agrochemicals in the market or research pipeline. Considering the vast abundance of these structural motifs, the development of chemical processes providing easy access to novel complex target molecules by introducing environmentally benign conditions with the main focus on improving the cost-effectiveness of the chemical transformation is highly demanding and challenging. Accordingly, sonochemistry appears to be an excellent alternative and a highly feasible environmentally benign energy input that has recently received considerable and steadily increasing interest in organic synthesis. However, the involvement of transition-metal-catalyst(s) in a chemical process often triggers an unintended impact on the greenness or sustainability of the transformation. Consequently, enormous efforts have been devoted to developing metal-free routes for assembling various heterocycles of medicinal interest, particularly under ultrasound irradiation. The present review article aims to demonstrate a brief overview of the current progress accomplished in the ultrasound-assisted synthesis of pharmaceutically relevant diverse heterocycles using transition-metal-free catalysis.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

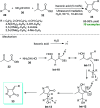
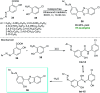
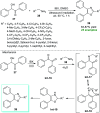
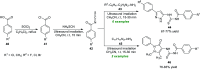

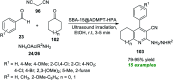
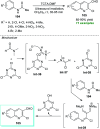
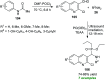
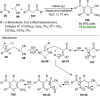

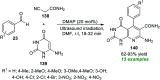
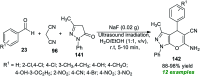
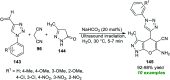
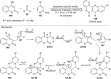
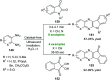
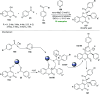
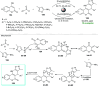
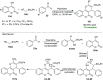
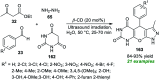
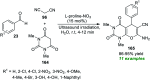
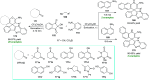
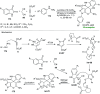
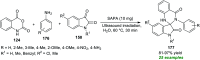


References
-
- Katritzky A. R. Chem. Heterocycl. Compd. 1992;28:241–259.
- Sahiba N. Agarwal S. Top. Curr. Chem. 2020;378:44. - PMC - PubMed
- Katritzky A. R., Ramsden C. A., Joule J. A. and Zhdankin V. V., Handbook of Heterocyclic Chemistry, Elsevier, 2010
- Hemming K. Annu. Rep. Prog. Chem., Sect. B: Org. Chem. 2009;105:129–149.
-
- Gomtsyan A. Chem. Heterocycl. Compd. 2012;48:7–10.
- Taylor A. P. Robinson R. P. Fobian Y. M. Blakemore D. C. Jones L. H. Fadeyi O. Org. Biomol. Chem. 2016;14:6611–6637. - PubMed
-
- Majumdar K. C. and Chattopadhyay S. K., Heterocycles in Natural Product Synthesis, John Wiley & Sons, 2011
- Joule J. A. Adv. Heterocycl. Chem. 2016;119:81–106.
-
- Cossy J. Guerinot A. Adv. Heterocycl. Chem. 2016;119:107–142.
-
- Cooke G. Evans I. R. Skabara P. J. J. Mater. Chem. C. 2019;7:6492.
Publication types
LinkOut - more resources
Full Text Sources

