Ion transport through a nanoporous C2N membrane: the effect of electric field and layer number
- PMID: 35558907
- PMCID: PMC9088869
- DOI: 10.1039/c8ra07795a
Ion transport through a nanoporous C2N membrane: the effect of electric field and layer number
Abstract
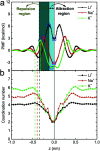
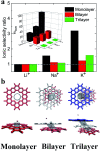
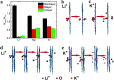
Ion transport through a two-dimensional membrane with nanopores plays an important role in many scientific and technical applications (e.g., water desalination, ion separation and nanofiltration). Although there have been many two-dimensional membranes for these applications, the problem of how to controllably fabricate nanopores with proper shape and size still remains challenging. In the present work, the transport of ions through a C2N membrane with intrinsically regular and uniformly distributed nanopores is investigated using all-atom molecular dynamic simulations. It was found that the monolayer C2N membrane possesses higher ion permeability compared to the graphene membrane because of its higher density of nanopores. In addition, it exhibits excellent ion selectivity under a low electric field due to the distinct dehydration capabilities and interaction behaviors (with the pore edges) of the different ions. Furthermore, we found that multilayer C2N membranes have weak ion selectivity, but show promising potential for desalination. The present study may provide some physical insights into the experimental design of C2N-based nanodevices in nanofluids.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- Wang L. Y. Wu H. A. Wang F. C. RSC Adv. 2017;7:20360–20368. doi: 10.1039/C7RA00890B. - DOI
LinkOut - more resources
Full Text Sources

