Construction of flower-like MoS2/Fe3O4/rGO composite with enhanced photo-Fenton like catalyst performance
- PMID: 35558923
- PMCID: PMC9088837
- DOI: 10.1039/c8ra06537c
Construction of flower-like MoS2/Fe3O4/rGO composite with enhanced photo-Fenton like catalyst performance
Abstract
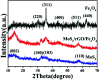
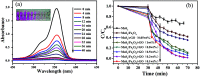
High-performance and recyclable photocatalysts have attracted considerable amounts of attention for use in wastewater treatment. In this paper, a MoS2/Fe3O4/rGO (0.1 wt%) composite was synthesized by an environmentally-friendly and facile strategy, and showed high potential for recyclability. The nanocomposite exhibited high photocatalytic activity in the presence of H2O2 and rGO (reduced graphene oxide) under visible-light irradiation. Notably, when 3 mg of MoS2/Fe3O4/rGO (0.1 wt%) was added to rhodamine B (RhB, 30 mg L-1) solution, the degradation rate was almost 100% within 40 min at neutral pH under visible-light irradiation. This rate was four times more rapid than that of MoS2 and double that of MoS2/Fe3O4. The results indicate that rGO plays an important role in photocatalysis by suppressing the recombination of photogenerated electron-hole pairs and enhancing the absorption capability of visible-light and organic dyes. Finally, the photocatalytic and stability mechanisms of MoS2/Fe3O4/rGO (0.1 wt%) are proposed. This work further helps our understanding of the photo-Fenton mechanism. Furthermore, the synthesis of this composite has potential for application in energy storage devices.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures







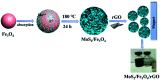
References
-
- Dias E. M. Petit C. J. Mater. Chem. A. 2015;3:22484–22506. doi: 10.1039/C5TA05440K. - DOI
-
- Cao C. Xiao L. Chen C. Cao Q. Appl. Surf. Sci. 2015;333:110–118. doi: 10.1016/j.apsusc.2015.02.002. - DOI
-
- Liang L. Zhu Q. Wang T. Wang F. Ma J. Jing L. Sun J. Microporous Mesoporous Mater. 2014;197:221–228. doi: 10.1016/j.micromeso.2014.06.025. - DOI
-
- Kim J. S. Kim B. Kim H. Kang K. Adv. Energy Mater. 2018;8:1702774. doi: 10.1002/aenm.201702774. - DOI
-
- Choe J. Y. Byun J. Y. Kim S. H. Appl. Catal., B. 2018;233:272–280. doi: 10.1016/j.apcatb.2018.03.110. - DOI
LinkOut - more resources
Full Text Sources

