Unorthodox synthesis, biological activity and DFT studies of novel and multifunctionalized naphthoxocine derivatives
- PMID: 35558993
- PMCID: PMC9088745
- DOI: 10.1039/c9ra05154f
Unorthodox synthesis, biological activity and DFT studies of novel and multifunctionalized naphthoxocine derivatives
Abstract
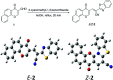
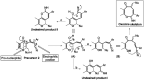
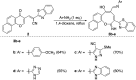
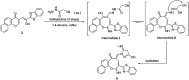
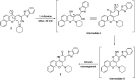
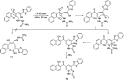
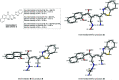
A new promising protocol has been developed for the synthesis of scarce oxocine derivatives 3a-e and 6 through addition of amine-based nucleophiles such as hydroxylamine hydrochloride, primary amine and hydrazide to chromonylidene benzothiazol-2-ylacetonitrile 2 in refluxing dioxane under metal free reaction conditions in moderate to good yields. Other nitrogen nucleophiles such as piperidine, hydrazine and thiosemicarbazide failed to afford the corresponding oxocinols, and instead pyridine derivatives 7, 8 and 10 were obtained exclusively. Predictive study for the biological activities using PASS (prediction of activity spectra for biologically active substances) online software showed optimistic activities for oxocinols 3a-e in the treatment of cancer, influenza A and microbial infections. Additionally, DFT studies of oxocine derivatives 3a-e and 6 indicated the presence of required thermodynamics parameters for the application in dye-sensitized solar cells (DSSCs).
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors have no conflict of interest to mention.
Figures
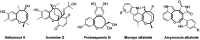


References
-
- Cossy J., Brimble M. and Cordes M., Synthesis of Saturated Oxygenated Heterocycles, Springer, 2014
-
- Steglich W. Pure Appl. Chem. 1989;61:281–288.
LinkOut - more resources
Full Text Sources

