Development and Validation of a Novel Gene Signature for Predicting the Prognosis of Idiopathic Pulmonary Fibrosis Based on Three Epithelial-Mesenchymal Transition and Immune-Related Genes
- PMID: 35559024
- PMCID: PMC9086533
- DOI: 10.3389/fgene.2022.865052
Development and Validation of a Novel Gene Signature for Predicting the Prognosis of Idiopathic Pulmonary Fibrosis Based on Three Epithelial-Mesenchymal Transition and Immune-Related Genes
Abstract
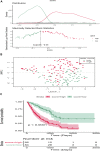
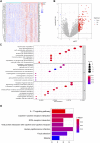
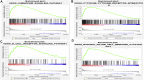
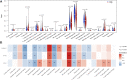
Background: Increasing evidence has revealed that epithelial-mesenchymal transition (EMT) and immunity play key roles in idiopathic pulmonary fibrosis (IPF). However, correlation between EMT and immune response and the prognostic significance of EMT in IPF remains unclear. Methods: Two microarray expression profiling datasets (GSE70866 and GSE28221) were downloaded from the Gene Expression Omnibus (GEO) database. EMT- and immune-related genes were identified by gene set variation analysis (GSVA) and the Estimation of STromal and Immune cells in MAlignant Tumors using Expression data (ESTIMATE) algorithm. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were performed to investigate the functions of these EMT- and immune-related genes. Cox and least absolute shrinkage and selection operator (LASSO) regression analyses were used to screen prognostic genes and establish a gene signature. Gene Set Enrichment Analysis (GSEA) and Cell-type Identification By Estimating Relative Subsets Of RNA Transcripts (CIBERSORT) were used to investigate the function of the EMT- and immune-related signatures and correlation between the EMT- and immune-related signatures and immune cell infiltration. Quantitative real-time polymerase chain reaction (qRT-PCR) was performed to investigate the mRNA expression of genes in the EMT- and immune-related signatures. Results: Functional enrichment analysis suggested that these genes were mainly involved in immune response. Moreover, the EMT- and immune-related signatures were constructed based on three EMT- and immune-related genes (IL1R2, S100A12, and CCL8), and the K-M and ROC curves presented that the signature could affect the prognosis of IPF patients and could predict the 1-, 2-, and 3-year survival well. Furthermore, a nomogram was developed based on the expression of IL1R2, S100A12, and CCL8, and the calibration curve showed that the nomogram could visually and accurately predict the 1-, 2-, 3-year survival of IPF patients. Finally, we further found that immune-related pathways were activated in the high-risk group of patients, and the EMT- and immune-related signatures were associated with NK cells activated, macrophages M0, dendritic cells resting, mast cells resting, and mast cells activated. qRT-PCR suggested that the mRNA expression of IL1R2, S100A12, and CCL8 was upregulated in whole blood of IPF patients compared with normal samples. Conclusion: IL1R2, S100A12, and CCL8 might play key roles in IPF by regulating immune response and could be used as prognostic biomarkers of IPF.
Keywords: epithelial–mesenchymal transition; idiopathic pulmonary fibrosis; immune; marker; prognosis.
Copyright © 2022 Zheng, Dong, Zhang, Ning, Xu and Cai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

