An efficient and ecofriendly synthesis of highly functionalized pyridones via a one-pot three-component reaction
- PMID: 35559211
- PMCID: PMC9092425
- DOI: 10.1039/c8ra05690k
An efficient and ecofriendly synthesis of highly functionalized pyridones via a one-pot three-component reaction
Abstract
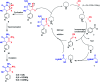
A simple and convenient protocol has been developed for the synthesis of N-amino-3-cyano-2-pyridone derivatives by a one-pot reaction of cyanoacetohydrazide, activated nitrile substrates (malononitrile, ethyl cyanoacetate, cyanoacetamide) and aromatic aldehydes in the presence of piperidine in water or a mixture of water and ethanol. The sequence of cascade reactions includes Knoevenagel condensation, Michael addition, intramolecular cyclization, imine-enamine tautomerization and oxidative aromatization. The main advantages of this procedure are availability of starting compounds, simple procedure, mild conditions, easy purification of products and the use of water or water/ethanol as green solvents.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Synthesis of highly functionalized thiazolo[3,2-a]pyridine derivatives via a five-component cascade reaction based on nitroketene N,S-acetal.RSC Adv. 2020 Aug 21;10(52):31039-31048. doi: 10.1039/d0ra03910a. eCollection 2020 Aug 21. RSC Adv. 2020. PMID: 35520681 Free PMC article.
-
A Novel Synthesis of Highly Functionalized Pyridines by a One-Pot, Three-Component Tandem Reaction of Aldehydes, Malononitrile and N-Alkyl-2-cyanoacetamides under Microwave Irradiation.Molecules. 2018 Mar 9;23(3):619. doi: 10.3390/molecules23030619. Molecules. 2018. PMID: 29522435 Free PMC article.
-
An efficient synthesis of new imidazo[1,2-a]pyridine-6-carbohydrazide and pyrido[1,2-a]pyrimidine-7-carbohydrazide derivatives via a five-component cascade reaction.RSC Adv. 2019 Mar 5;9(13):7218-7227. doi: 10.1039/c9ra00350a. eCollection 2019 Mar 1. RSC Adv. 2019. PMID: 35519992 Free PMC article.
-
Synthesis of 5-amino-N'-(9H-fluoren-9-ylidene)-8-nitro-7-aryl-1,2,3,7-tetrahydroimidazo[1,2-a]pyridine-6-carbohydrazide derivatives based on heterocyclic ketene aminals.RSC Adv. 2018 Dec 11;8(72):41218-41225. doi: 10.1039/c8ra09308c. eCollection 2018 Dec 7. RSC Adv. 2018. PMID: 35559289 Free PMC article.
-
Efficient regioselective five-component synthesis of novel thiazolo[3,2-a]pyridine carbohydrazides and oxazolo[3,2-a]pyridine carbohydrazides.Mol Divers. 2023 Apr;27(2):667-678. doi: 10.1007/s11030-022-10446-0. Epub 2022 May 19. Mol Divers. 2023. PMID: 35587848
Cited by
-
Synthesis of Metal-Organic Frameworks MIL-101(Cr)-NH2 Containing Phosphorous Acid Functional Groups: Application for the Synthesis of N-Amino-2-pyridone and Pyrano [2,3-c]pyrazole Derivatives via a Cooperative Vinylogous Anomeric-Based Oxidation.ACS Omega. 2020 Mar 20;5(12):6240-6249. doi: 10.1021/acsomega.9b02133. eCollection 2020 Mar 31. ACS Omega. 2020. PMID: 32258858 Free PMC article.
-
A highly chemo- and regioselective synthesis of heterocyclic [3.3.3]propellanes via sequential multicomponent reactions in water.Sci Rep. 2025 Jul 31;15(1):27969. doi: 10.1038/s41598-025-13577-0. Sci Rep. 2025. PMID: 40745019 Free PMC article.
-
Multicomponent synthesis of novel functionalized spiroindenopyridotriazine-4H-pyrans.RSC Adv. 2025 Mar 5;15(9):7103-7110. doi: 10.1039/d5ra01048a. eCollection 2025 Feb 26. RSC Adv. 2025. PMID: 40045953 Free PMC article.
-
Efficient synthesis of new indenopyridotriazine [4.3.3]propellanes and spiroindenopyridotriazine-4H-pyran derivatives.RSC Adv. 2023 Oct 27;13(45):31488-31496. doi: 10.1039/d3ra06248a. eCollection 2023 Oct 26. RSC Adv. 2023. PMID: 37901267 Free PMC article.
-
A one-pot synthesis of piperidinium spirooxindoline-pyridineolates and indole-substituted pyridones in aqueous or ethanol medium.Mol Divers. 2022 Aug;26(4):2039-2048. doi: 10.1007/s11030-021-10313-4. Epub 2021 Sep 15. Mol Divers. 2022. PMID: 34528212
References
-
- Benson S. C. Gross J. L. Snyder J. K. J. Org. Chem. 1990;55:3257–3269. doi: 10.1021/jo00297a050. - DOI
-
- Thomass A. Chakraborty M. Junjappa H. Tetrahedron. 1990;46:577–586. doi: 10.1016/S0040-4020(01)85438-7. - DOI
-
- Wolff J. Taddei M. Tetrahedron. 1986;42:4267–4272. doi: 10.1016/S0040-4020(01)87652-3. - DOI
-
- Taylor E. C. J. Heterocycl. Chem. 1990;27:1–12. doi: 10.1002/jhet.5570270101. - DOI
-
- Tominaga Y. Kohra S. Honkawa H. Hosomi A. Heterocycles. 1989;28:1409. doi: 10.3987/REV-89-408. - DOI
LinkOut - more resources
Full Text Sources

