Tetrathienothiophene Porphyrin as a Metal-Free Sensitizer for Room-Temperature Triplet-Triplet Annihilation Upconversion
- PMID: 35559213
- PMCID: PMC9086237
- DOI: 10.3389/fchem.2022.809863
Tetrathienothiophene Porphyrin as a Metal-Free Sensitizer for Room-Temperature Triplet-Triplet Annihilation Upconversion
Abstract
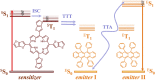
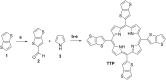
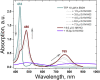
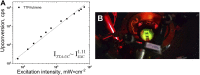
Optically excited triplet states of organic molecules serve as an energy pool for the subsequent processes, either photon energy downhill, such as room temperature phosphorescence, or photon energy uphill process-the triplet-triplet annihilation upconversion (TTA-UC). Manifestation of a high intersystem crossing coefficient is an unavoidable requirement for triplet state formation, following the absorption of a single photon. This requirement is even more inevitable if the excitation light is non-coherent, with moderate intensity and extremely low spectral power density, when compared with the light parameters of 1 Sun (1.5 AM). Coordination of a heavy atom increases substantially the probability of intersystem crossing. Nevertheless, having in mind the global shortage in precious and rare-earth metals, identification of metal-free organic moieties able to form triplet states becomes a prerequisite for environmental friendly optoelectronic technologies. This motivates us to synthesize a metal-free thienothiophene containing porphyrin, based on a condensation reaction between thienothiophene-2-carbaldehyde and pyrrole in an acidic medium by modified synthetic protocol. The upconversion couple tetrathienothiophene porphyrin/rubrene when excited at λ = 658 nm demonstrates bright, delayed fluorescence with a maximum emission at λ = 555 nm. This verifies our hypothesis that the ISC coefficient in thienothiophene porphyrin is efficient in order to create even at room temperature and low-intensity optical excitation densely populated organic triplet ensemble and is suitable for photon energy uphill processes, which makes this type of metal-free sensitizers even more important for optoelectronic applications.
Keywords: acidification; inter system crossing; metal-free triplet–triplet annihilation; room temperature; triplet state formation.
Copyright © 2022 Vasilev, Kostadinov, Kandinska, Landfester and Baluschev.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Recent Advances in the Photoreactions Triggered by Porphyrin-Based Triplet-Triplet Annihilation Upconversion Systems: Molecular Innovations and Nanoarchitectonics.Int J Mol Sci. 2022 Jul 21;23(14):8041. doi: 10.3390/ijms23148041. Int J Mol Sci. 2022. PMID: 35887385 Free PMC article. Review.
-
New Triplet Sensitization Routes for Photon Upconversion: Thermally Activated Delayed Fluorescence Molecules, Inorganic Nanocrystals, and Singlet-to-Triplet Absorption.Acc Chem Res. 2017 Oct 17;50(10):2487-2495. doi: 10.1021/acs.accounts.7b00235. Epub 2017 Sep 20. Acc Chem Res. 2017. PMID: 28930435
-
BODIPY-pyrene donor-acceptor sensitizers for triplet-triplet annihilation upconversion: the impact of the BODIPY-core on upconversion efficiency.Phys Chem Chem Phys. 2022 Feb 9;24(6):3568-3578. doi: 10.1039/d1cp05382e. Phys Chem Chem Phys. 2022. PMID: 35084007
-
Pd-Porphyrin Oligomers Sensitized for Green-to-Blue Photon Upconversion: The More the Better?Chemistry. 2016 Jun 13;22(25):8654-62. doi: 10.1002/chem.201504498. Epub 2016 May 3. Chemistry. 2016. PMID: 27143644
-
Triplet-triplet annihilation-based photon upconversion using nanoparticles and nanoclusters.Mater Horiz. 2024 May 20;11(10):2304-2322. doi: 10.1039/d4mh00117f. Mater Horiz. 2024. PMID: 38587491 Review.
Cited by
-
Recent Advances in the Photoreactions Triggered by Porphyrin-Based Triplet-Triplet Annihilation Upconversion Systems: Molecular Innovations and Nanoarchitectonics.Int J Mol Sci. 2022 Jul 21;23(14):8041. doi: 10.3390/ijms23148041. Int J Mol Sci. 2022. PMID: 35887385 Free PMC article. Review.
-
Recent synthetic approaches towards thienothiophenes: a potential template for biologically active compounds.Mol Divers. 2024 Jun;28(3):1793-1821. doi: 10.1007/s11030-023-10647-1. Epub 2023 Apr 24. Mol Divers. 2024. PMID: 37095354 Review.
References
-
- Askes S. H. C., Bonnet S. (2018). Solving the Oxygen Sensitivity of Sensitized Photon Upconversion in Life Science Applications. Nat. Rev. Chem. 2 (12), 437–452. 10.1038/s41570-018-0057-z - DOI
-
- Askes S. H. C., Leeuwenburgh V. C., Pomp W., Arjmandi-Tash H., Tanase S., Schmidt T., et al. (2017). Water-Dispersible Silica-Coated Upconverting Liposomes: Can a Thin Silica Layer Protect TTA-UC against Oxygen Quenching? ACS Biomater. Sci. Eng. 3, 322–334. 10.1021/acsbiomaterials.6b00678 - DOI - PMC - PubMed
-
- Baluschev S., Katta K., Avlasevich Y., Landfester K. (2016). Annihilation Upconversion in Nanoconfinement: Solving the Oxygen Quenching Problem. Mater. Horiz. 3, 478–486. 10.1039/c6mh00289g - DOI
-
- Bhyrappa P., Bhavana P. (2001). Meso-tetrathienylporphyrins: Electrochemical and Axial Ligation Properties. Chem. Phys. Lett. 349, 399–404. 10.1016/S0009-2614(01)01189-7 - DOI
LinkOut - more resources
Full Text Sources

