Adolescent Binge Alcohol Enhances Early Alzheimer's Disease Pathology in Adulthood Through Proinflammatory Neuroimmune Activation
- PMID: 35559229
- PMCID: PMC9086457
- DOI: 10.3389/fphar.2022.884170
Adolescent Binge Alcohol Enhances Early Alzheimer's Disease Pathology in Adulthood Through Proinflammatory Neuroimmune Activation
Abstract
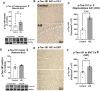
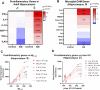
Epidemiological studies suggest that heavy alcohol use early in life is associated with increased risk for Alzheimer's disease (AD). However, mechanisms connecting AD with alcohol use have not been identified. Both heavy alcohol use and AD feature increased proinflammatory signaling. Therefore, we hypothesized that adolescent binge ethanol would increase AD molecular and behavioral pathology in adulthood through proinflammatory signaling. The 3xTg-AD mouse model (APPSwe, tauP301, Psen1tm1Mpm) which features amyloid (Aβ) and tau pathology beginning at 6-12 months underwent adolescent intermittent ethanol (AIE, 5 g/kg/d, i.g., P25-55) with assessment of AD pathologic mediators at P200. A second group of mice received AIE +/- minocycline (30 mg/kg/d, IP) followed by behavioral testing in adulthood. Behavioral testing and age of testing included: locomotor activity and exploration (27-28 weeks), novel object recognition (NORT, 28-30 weeks), 3-chamber sociability and social memory (29-31 weeks), prepulse inhibition (PPI, 30-32 weeks), Morris Water Maze with reversal (MWM, 31-35 weeks), and Piezo sleep monitoring (35-37 weeks). We found that AIE increased levels of neurotoxic Aβ1-42 in adult female hippocampus as well as intraneuronal Aβ1-42 in amygdala and entorhinal cortex. Phosphorylated tau at residue Thr181 (p-tau-181) was also increased in female hippocampus by AIE. Several proinflammatory genes were persistently increased by AIE in the female hippocampus, including IL-1β, MCP-1, IL-6, and IFNα. Expression of these genes was strongly correlated with the levels of Aβ1-42 and p-tau-181 in hippocampus. AIE caused persistent decreases in locomotor activity (open-field and NORT habituation) and increased anxiety-like behavior (thigmotaxis) while reducing memory retention. Treatment with the anti-inflammatory compound minocycline during AIE blocked persistent increases in Aβ1-42 in amygdala and p-tau-181 in hippocampus, and prevented AIE-induced thigmotaxis and memory loss. Together, these data find that adolescent binge ethanol enhances AD molecular and behavioral pathology in adulthood through proinflammatory signaling. Blockade of proinflammatory signaling during ethanol exposure prevents ethanol-induced effects on pathologic accumulation of AD-associated proteins and persistent behavior changes relevant to human AD.
Keywords: Alzheiemer’s disease; addiction; adolescence; alcohol; amyloid; neuroinflammation.
Copyright © 2022 Barnett, David, Rohlman, Nikolova, Moy, Vetreno and Coleman.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Chronic Ethanol Causes Persistent Increases in Alzheimer's Tau Pathology in Female 3xTg-AD Mice: A Potential Role for Lysosomal Impairment.Front Behav Neurosci. 2022 May 11;16:886634. doi: 10.3389/fnbeh.2022.886634. eCollection 2022. Front Behav Neurosci. 2022. PMID: 35645744 Free PMC article.
-
Persistent Adult Neuroimmune Activation and Loss of Hippocampal Neurogenesis Following Adolescent Ethanol Exposure: Blockade by Exercise and the Anti-inflammatory Drug Indomethacin.Front Neurosci. 2018 Mar 28;12:200. doi: 10.3389/fnins.2018.00200. eCollection 2018. Front Neurosci. 2018. PMID: 29643762 Free PMC article.
-
Epigenetic regulation of microglia and neurons by proinflammatory signaling following adolescent intermittent ethanol (AIE) exposure and in human AUD.Adv Drug Alcohol Res. 2024 Mar 8;4:12094. doi: 10.3389/adar.2024.12094. eCollection 2024. Adv Drug Alcohol Res. 2024. PMID: 38524847 Free PMC article. Review.
-
Targeting Persistent Changes in Neuroimmune and Epigenetic Signaling in Adolescent Drinking to Treat Alcohol Use Disorder in Adulthood.Pharmacol Rev. 2023 Mar;75(2):380-396. doi: 10.1124/pharmrev.122.000710. Epub 2022 Dec 12. Pharmacol Rev. 2023. PMID: 36781218 Free PMC article. Review.
-
Neuroimmune and epigenetic involvement in adolescent binge ethanol-induced loss of basal forebrain cholinergic neurons: Restoration with voluntary exercise.Addict Biol. 2020 Mar;25(2):e12731. doi: 10.1111/adb.12731. Epub 2019 Feb 18. Addict Biol. 2020. PMID: 30779268 Free PMC article.
Cited by
-
Alcohol Use Disorder and Dementia: A Review.Alcohol Res. 2024 May 23;44(1):03. doi: 10.35946/arcr.v44.1.03. eCollection 2024. Alcohol Res. 2024. PMID: 38812709 Free PMC article. Review.
-
Biochemical changes precede affective and cognitive anomalies in aging adult C57BL/6J mice with a prior history of adolescent alcohol binge-drinking.Addict Biol. 2024 Dec;29(12):e70006. doi: 10.1111/adb.70006. Addict Biol. 2024. PMID: 39665499 Free PMC article.
-
Chronic alcohol exposure during young adulthood attenuates microglial reactivity and downstream immune response pathways in a mouse model of tauopathy later in life.Alcohol Clin Exp Res (Hoboken). 2025 May;49(5):985-1000. doi: 10.1111/acer.70034. Epub 2025 Mar 21. Alcohol Clin Exp Res (Hoboken). 2025. PMID: 40114609
-
Substance Abuse and Cognitive Decline: The Critical Role of Tau Protein as a Potential Biomarker.Int J Mol Sci. 2025 Aug 7;26(15):7638. doi: 10.3390/ijms26157638. Int J Mol Sci. 2025. PMID: 40806766 Free PMC article. Review.
-
Moderate ethanol exposure reduces astrocyte-induced neuroinflammatorysignaling and cognitive decline in presymptomatic APP/PS1 mice.Res Sq [Preprint]. 2023 Dec 2:rs.3.rs-3627637. doi: 10.21203/rs.3.rs-3627637/v1. Res Sq. 2023. Update in: Sci Rep. 2024 Oct 14;14(1):23989. doi: 10.1038/s41598-024-75202-w. PMID: 38077051 Free PMC article. Updated. Preprint.
References
-
- Attarzadeh-Yazdi G., Arezoomandan R., Haghparast A. (2014). Minocycline, an Antibiotic with Inhibitory Effect on Microglial Activation, Attenuates the Maintenance and Reinstatement of Methamphetamine-Seeking Behavior in Rat. Prog. Neuropsychopharmacol. Biol. Psychiatry 53, 142–148. 10.1016/j.pnpbp.2014.04.008 - DOI - PubMed
-
- Bekhbat M., Mukhara D., Dozmorov M. G., Stansfield J. C., Benusa S. D., Hyer M. M., et al. (2021). Adolescent Stress Sensitizes the Adult Neuroimmune Transcriptome and Leads to Sex-specific Microglial and Behavioral Phenotypes. Neuropsychopharmacology 46, 949–958. 10.1038/s41386-021-00970-2 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

