The Role and Mechanism of Lysine Methyltransferase and Arginine Methyltransferase in Kidney Diseases
- PMID: 35559246
- PMCID: PMC9086358
- DOI: 10.3389/fphar.2022.885527
The Role and Mechanism of Lysine Methyltransferase and Arginine Methyltransferase in Kidney Diseases
Abstract
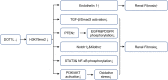
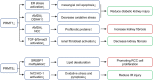
Methylation can occur in both histones and non-histones. Key lysine and arginine methyltransferases under investigation for renal disease treatment include enhancer of zeste homolog 2 (EZH2), G9a, disruptor of telomeric silencing 1-like protein (DOT1L), and protein arginine methyltransferases (PRMT) 1 and 5. Recent studies have shown that methyltransferases expression and activity are also increased in several animal models of kidney injury, such as acute kidney injury(AKI), obstructive nephropathy, diabetic nephropathy and lupus nephritis. The inhibition of most methyltransferases can attenuate kidney injury, while the role of methyltransferase in different animal models remains controversial. In this article, we summarize the role and mechanism of lysine methyltransferase and arginine methyltransferase in various kidney diseases and highlight methyltransferase as a potential therapeutic target for kidney diseases.
Keywords: acute kidney injury; arginine methyltransferase; chronic kidney diseases; epigenetic modification; lysine methyltransferase; renal cell carcinoma.
Copyright © 2022 Zhou, Chen, Li, Shi, Zhuang and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Histone Methyltransferase EZH2: A Potential Therapeutic Target for Kidney Diseases.Front Physiol. 2021 Feb 18;12:640700. doi: 10.3389/fphys.2021.640700. eCollection 2021. Front Physiol. 2021. PMID: 33679454 Free PMC article. Review.
-
Histone H3K27 methyltransferase EZH2 regulates apoptotic and inflammatory responses in sepsis-induced AKI.Theranostics. 2023 Mar 21;13(6):1860-1875. doi: 10.7150/thno.83353. eCollection 2023. Theranostics. 2023. PMID: 37064878 Free PMC article.
-
Chemogenetic analysis of human protein methyltransferases.Chem Biol Drug Des. 2011 Aug;78(2):199-210. doi: 10.1111/j.1747-0285.2011.01135.x. Epub 2011 Jun 16. Chem Biol Drug Des. 2011. PMID: 21564555
-
Multiple Histone Lysine Methyltransferases Are Required for the Establishment and Maintenance of HIV-1 Latency.mBio. 2017 Feb 28;8(1):e00133-17. doi: 10.1128/mBio.00133-17. mBio. 2017. PMID: 28246360 Free PMC article.
-
The diverse functions of Dot1 and H3K79 methylation.Genes Dev. 2011 Jul 1;25(13):1345-58. doi: 10.1101/gad.2057811. Genes Dev. 2011. PMID: 21724828 Free PMC article. Review.
Cited by
-
microRNA-29b-3p attenuates diabetic nephropathy in mice by modifying EZH2.Hormones (Athens). 2023 Jun;22(2):223-233. doi: 10.1007/s42000-022-00426-2. Epub 2023 Jan 24. Hormones (Athens). 2023. PMID: 36692688
-
Protein posttranslational modifications in health and diseases: Functions, regulatory mechanisms, and therapeutic implications.MedComm (2020). 2023 May 2;4(3):e261. doi: 10.1002/mco2.261. eCollection 2023 Jun. MedComm (2020). 2023. PMID: 37143582 Free PMC article. Review.
-
Osteoclasts and osteoarthritis: Novel intervention targets and therapeutic potentials during aging.Aging Cell. 2024 Apr;23(4):e14092. doi: 10.1111/acel.14092. Epub 2024 Jan 29. Aging Cell. 2024. PMID: 38287696 Free PMC article. Review.
-
A novel role of DOT1L in kidney diseases.Mol Biol Rep. 2023 Jun;50(6):5415-5423. doi: 10.1007/s11033-023-08415-3. Epub 2023 Apr 22. Mol Biol Rep. 2023. PMID: 37085741 Review.
-
DOT1L promotes cell proliferation and invasion by epigenetically regulating STAT5B in renal cell carcinoma.Am J Cancer Res. 2023 Jan 15;13(1):276-292. eCollection 2023. Am J Cancer Res. 2023. PMID: 36777512 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

