A fluorescent sensor constructed from nitrogen-doped carbon nanodots (N-CDs) for pH detection in synovial fluid and urea determination
- PMID: 35559313
- PMCID: PMC9091950
- DOI: 10.1039/c8ra08406h
A fluorescent sensor constructed from nitrogen-doped carbon nanodots (N-CDs) for pH detection in synovial fluid and urea determination
Abstract
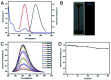
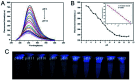
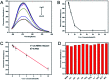
Blue luminescent nitrogen-doped carbon nanodots (N-CDs) with pH-dependent properties were prepared from citric acid (CA), glutathione (GSH), and polyethylene polyamine (PEPA) using a two-step pyrolytic route. The N-CDs showed stable and strong emission bands at approximately 455 nm under 350 nm excitation. Moreover, the fluorescence of N-CDs can be gradually decreased by gradually increasing the pH value. A good linear relationship between the fluorescence intensity of N-CDs and the pH range of 3.0-9.0 was obtained. Thus, the response mechanism of N-CDs to pH was systematically investigated. N-CDs possessed -NH2, -COOH, and -CONH- as active functional groups, which allowed the variable protonation/deprotonation of N-CDs to regulate the fluorescence emission intensities under changed pH values. Furthermore, upon combining urease-catalyzed hydrolysis of urea with increased pH values, a simple but effective fluorescence assay for urea was developed. The analytical performance for urea detection was the linear range of 0 to 10 mM with a detection limit of 0.072 mM. Additionally, the fluorescent sensor based on N-CDs was successfully applied for pH detection in synovial fluid and urea determination in serum.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
-
- Demaurex N. News Physiol. Sci. 2002;17:1. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

