FasL microgels induce immune acceptance of islet allografts in nonhuman primates
- PMID: 35559682
- PMCID: PMC9106299
- DOI: 10.1126/sciadv.abm9881
FasL microgels induce immune acceptance of islet allografts in nonhuman primates
Abstract
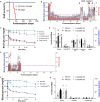
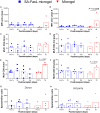
Islet transplantation to treat insulin-dependent diabetes is greatly limited by the need for maintenance immunosuppression. We report a strategy through which cotransplantation of allogeneic islets and streptavidin (SA)-FasL-presenting microgels to the omentum under transient rapamycin monotherapy resulted in robust glycemic control, sustained C-peptide levels, and graft survival in diabetic nonhuman primates for >6 months. Surgical extraction of the graft resulted in prompt hyperglycemia. In contrast, animals receiving microgels without SA-FasL under the same rapamycin regimen rejected islet grafts acutely. Graft survival was associated with increased number of FoxP3+ cells in the graft site with no significant changes in T cell systemic frequencies or responses to donor and third-party antigens, indicating localized tolerance. Recipients of SA-FasL microgels exhibited normal liver and kidney metabolic function, demonstrating safety. This localized immunomodulatory strategy succeeded with unmodified islets and does not require long-term immunosuppression, showing translational potential in β cell replacement for treating type 1 diabetes.
Figures



References
-
- Thompson D. M., Meloche M., Ao Z., Paty B., Keown P., Shapiro R. J., Ho S., Worsley D., Fung M., Meneilly G., Begg I., Al Mehthel M., Kondi J., Harris C., Fensom B., Kozak S. E., Tong S. O., Trinh M., Warnock G. L., Reduced progression of diabetic microvascular complications with islet cell transplantation compared with intensive medical therapy. Transplantation 91, 373–378 (2011). - PubMed
-
- Shapiro A. M., Geng Hao E., Lakey J. R., Finegood D. T., Rajotte R. V., Kneteman N. M., Defining optimal immunosuppression for islet transplantation based on reduced diabetogenicity in canine islet autografts. Transplantation 74, 1522–1528 (2002). - PubMed
-
- Billingham R. E., Brent L., Medawar P. B., Actively acquired tolerance of foreign cells. Nature 172, 603–606 (1953). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

