Exogenous insulin-like growth factor 1 accelerates growth and maturation of follicles in human cortical xenografts and increases ovarian output in mice
- PMID: 35560275
- PMCID: PMC9361175
- DOI: 10.1016/j.xfss.2021.07.002
Exogenous insulin-like growth factor 1 accelerates growth and maturation of follicles in human cortical xenografts and increases ovarian output in mice
Abstract
Objective: To measure the influence of exogenous insulin-like growth factor 1 (IGF1) on follicle growth and maturation in human ovarian cortical xenografts.
Design: Xenotransplantation model.
Setting: University-based research laboratory.
Patients/animals: Ovarian tissue was donated with consent and institutional review board approval by brain-dead organ donors or patients undergoing ovarian tissue cryopreservation for fertility preservation. Cortical fragments were transplanted into immunocompromised mice.
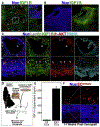
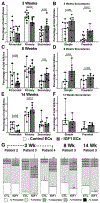
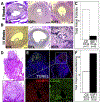
Interventions: Cryopreserved ovarian cortical fragments from four women (aged 19, 25, 33, and 46 years) were transplanted into the gluteus muscle of immunocompromised mice in a fibrin matrix containing endothelial cells that were transduced with lentiviral particles encoding secreted IGF1. Xenografts were recovered after 3, 8, and 14 weeks. In addition, C57/Bl6 mice underwent intraovarian injection of saline or recombinant IGF1 (60 μg), followed by superovulation, analysis of ethynyl-deoxyuridine incorporation, and ribonucleic acid sequencing of the whole ovaries.
Main outcome measures: For xenografts: follicle count and distribution; antral follicle count; and corpora lutea/albicans count. For mice: follicle count and distribution; oocyte yield, ethynyl-deoxyuridine incorporation (granulosa cell proliferation); and ovarian transcriptomic signature.
Results: At 3 weeks, xenografts in the IGF1 condition revealed a decreased percentage of primary follicles and increased percentage of secondary follicles that were concentrated in the preantral subtype; at 8 weeks, an increase in secondary follicles was concentrated in the simple subtype; after 14 weeks, primordial follicles were reduced, and while the number of advanced follicles did not power the experiment to demonstrate significance, antral follicles reduced and corpora lutea increased. Supporting experiments in mice revealed an increase in normal oocytes following intraovarian injection of recombinant IGF1 (60 μg) as well as increased proliferative index among follicles of secondary and preantral stages. Ribonucleic acid sequencing analysis of the whole ovaries following injection of recombinant IGF1 (25 μg) revealed an acute (24 hours) upregulation of transcripts related to steroidogenesis and luteinization.
Conclusions: Exogenous IGF1 advances the pace of growth among primordial, primary, and secondary stage follicles but results in near absence of antral stage follicles in long-term (14 weeks) xenografts. In mice, acute administration of IGF1 promotes follicle advance and increased oocyte yield. The results suggest that while superphysiological IGF1 alone advances the pace of growth among early/preantral follicles, a sustained and/or later-stage influence undermines antral follicle growth/survival or promotes premature luteinization. These findings provide a temporal framework for interpreting follicle growth/mobilization and may be useful in understanding the clinical application of human growth hormone in the context of assisted reproduction.
Keywords: Insulin-like growth factor 1; endothelial cell cotransplantation; folliculogenesis; growth hormone; human cortical xenograft.
Copyright © 2021 American Society for Reproductive Medicine. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Chronic superphysiologic AMH promotes premature luteinization of antral follicles in human ovarian xenografts.Sci Adv. 2022 Mar 11;8(10):eabi7315. doi: 10.1126/sciadv.abi7315. Epub 2022 Mar 9. Sci Adv. 2022. PMID: 35263130 Free PMC article.
-
Matrix-free 3D culture supports human follicular development from the unilaminar to the antral stage in vitro yielding morphologically normal metaphase II oocytes.Hum Reprod. 2021 Apr 20;36(5):1326-1338. doi: 10.1093/humrep/deab003. Hum Reprod. 2021. PMID: 33681988 Free PMC article.
-
Fibrin promotes development and function of macaque primary follicles during encapsulated three-dimensional culture.Hum Reprod. 2013 Aug;28(8):2187-200. doi: 10.1093/humrep/det093. Epub 2013 Apr 21. Hum Reprod. 2013. PMID: 23608357 Free PMC article.
-
Review: Roles of follicle-stimulating hormone in preantral folliculogenesis of domestic animals: what can we learn from model species and where do we go from here?Animal. 2023 May;17 Suppl 1:100743. doi: 10.1016/j.animal.2023.100743. Animal. 2023. PMID: 37567683 Review.
-
Intraovarian control of early folliculogenesis.Endocr Rev. 2015 Feb;36(1):1-24. doi: 10.1210/er.2014-1020. Epub 2014 Sep 9. Endocr Rev. 2015. PMID: 25202833 Free PMC article. Review.
Cited by
-
Thawing fertility: a view of ovarian tissue cryopreservation processes and review of ovarian transplant research.Fertil Steril. 2024 Oct;122(4):574-585. doi: 10.1016/j.fertnstert.2024.07.005. Epub 2024 Jul 9. Fertil Steril. 2024. PMID: 38992745 Review.
-
Chronic superphysiologic AMH promotes premature luteinization of antral follicles in human ovarian xenografts.Sci Adv. 2022 Mar 11;8(10):eabi7315. doi: 10.1126/sciadv.abi7315. Epub 2022 Mar 9. Sci Adv. 2022. PMID: 35263130 Free PMC article.
-
Molecular crosstalk between insulin-like growth factors and follicle-stimulating hormone in the regulation of granulosa cell function.Reprod Med Biol. 2024 Apr 3;23(1):e12575. doi: 10.1002/rmb2.12575. eCollection 2024 Jan-Dec. Reprod Med Biol. 2024. PMID: 38571513 Free PMC article. Review.
-
Xenograft model of heterotopic transplantation of human ovarian cortical tissue and its clinical relevance.Reproduction. 2022 Dec 7;165(1):31-47. doi: 10.1530/REP-22-0114. Print 2023 Jan 1. Reproduction. 2022. PMID: 36194429 Free PMC article.
References
-
- Salmon WD Jr, Daughaday WH. A hormonally controlled serum factor which stimulates sulfate incorporation by cartilage in vitro. J Lab Clin Med 1957;49: 825–36. - PubMed
-
- Daughaday WH, Hall K, Raben MS, Salmon WD Jr, van den Brande JL, van Wyk JJ. Somatomedin: proposed designation for sulphation factor. Nature 1972;235:107. - PubMed
-
- Hakuno F, Takahashi SI. IGF1 receptor signaling pathways. J Mol Endocrinol 2018;61:T69–86. - PubMed
-
- Guarente L, Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature 2000;408:255–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

