Cine gastric MRI reveals altered Gut-Brain Axis in Functional Dyspepsia: gastric motility is linked with brainstem-cortical fMRI connectivity
- PMID: 35560690
- PMCID: PMC9529794
- DOI: 10.1111/nmo.14396
Cine gastric MRI reveals altered Gut-Brain Axis in Functional Dyspepsia: gastric motility is linked with brainstem-cortical fMRI connectivity
Abstract
Background: Functional dyspepsia (FD) is a disorder of gut-brain interaction, and its putative pathophysiology involves dysregulation of gastric motility and central processing of gastric afference. The vagus nerve modulates gastric peristalsis and carries afferent sensory information to brainstem nuclei, specifically the nucleus tractus solitarii (NTS). Here, we combine MRI assessment of gastric kinematics with measures of NTS functional connectivity to the brain in patients with FD and healthy controls (HC), in order to elucidate how gut-brain axis communication is associated with FD pathophysiology.
Methods: Functional dyspepsia and HC subjects experienced serial gastric MRI and brain fMRI following ingestion of a food-based contrast meal. Gastric function indices estimated from 4D cine MRI data were compared between FD and HC groups using repeated measure ANOVA models, controlling for ingested volume. Brain connectivity of the NTS was contrasted between groups and associated with gastric function indices.
Key results: Propagation velocity of antral peristalsis was significantly lower in FD compared to HC. The brain network defined by NTS connectivity loaded most strongly onto the Default Mode Network, and more strongly onto the Frontoparietal Network in FD. FD also demonstrated higher NTS connectivity to insula, anterior cingulate and prefrontal cortices, and pre-supplementary motor area. NTS connectivity was linked to propagation velocity in HC, but not FD, whereas peristalsis frequency was linked with NTS connectivity in patients with FD.
Conclusions & inferences: Our multi-modal MRI approach revealed lower peristaltic propagation velocity linked to altered brainstem-cortical functional connectivity in patients suffering from FD suggesting specific plasticity in gut-brain communication.
Keywords: MRI; functional dyspepsia; interoception; stomach; vagus nerve.
© 2022 John Wiley & Sons Ltd.
Conflict of interest statement
Figures

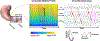
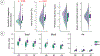
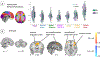

Comment in
-
Technical advances allow in-depth understanding of the gut-brain interaction-yet important caveats remain.Neurogastroenterol Motil. 2022 Nov;34(11):e14446. doi: 10.1111/nmo.14446. Epub 2022 Aug 9. Neurogastroenterol Motil. 2022. PMID: 35946060 No abstract available.
-
Response to "Technical advances allow in-depth understanding of the gut-brain interaction - yet important caveats remain".Neurogastroenterol Motil. 2022 Nov;34(11):e14460. doi: 10.1111/nmo.14460. Epub 2022 Sep 14. Neurogastroenterol Motil. 2022. PMID: 36102629 No abstract available.
Similar articles
-
Evaluation of Gastric Peristalsis Using Cine MRI in Healthy Subjects and Patients With Functional Dyspepsia.Clin Transl Gastroenterol. 2025 Aug 4. doi: 10.14309/ctg.0000000000000900. Online ahead of print. Clin Transl Gastroenterol. 2025. PMID: 40758175
-
Disrupted intrinsic connectivity of the periaqueductal gray in patients with functional dyspepsia: A resting-state fMRI study.Neurogastroenterol Motil. 2017 Aug;29(8). doi: 10.1111/nmo.13060. Epub 2017 Mar 24. Neurogastroenterol Motil. 2017. PMID: 28338267
-
Altered structural and functional connectivity of the insula in functional dyspepsia.Neurogastroenterol Motil. 2018 Sep;30(9):e13345. doi: 10.1111/nmo.13345. Epub 2018 Apr 23. Neurogastroenterol Motil. 2018. PMID: 29687532
-
Functional dyspepsia assessment - current diagnostic methods and new promising techniques.Arch Clin Cases. 2025 Mar 19;12(1):37-43. doi: 10.22551/2025.46.1201.10310. eCollection 2025. Arch Clin Cases. 2025. PMID: 40110365 Free PMC article. Review.
-
Ghrelin: a gut hormonal basis of motility regulation and functional dyspepsia.J Gastroenterol Hepatol. 2011 Apr;26 Suppl 3:67-72. doi: 10.1111/j.1440-1746.2011.06630.x. J Gastroenterol Hepatol. 2011. PMID: 21443713 Review.
Cited by
-
A novel compartmental approach for modeling stomach motility and gastric emptying.Comput Biol Med. 2024 Oct;181:109035. doi: 10.1016/j.compbiomed.2024.109035. Epub 2024 Aug 29. Comput Biol Med. 2024. PMID: 39213708
-
Diffeomorphic Surface Modeling for MRI-Based Characterization of Gastric Anatomy and Motility.IEEE Trans Biomed Eng. 2023 Jul;70(7):2046-2057. doi: 10.1109/TBME.2023.3234509. Epub 2023 Jun 20. IEEE Trans Biomed Eng. 2023. PMID: 37018592 Free PMC article.
-
Interoceptive Processing in Functional Gastrointestinal Disorders.Int J Mol Sci. 2024 Jul 11;25(14):7633. doi: 10.3390/ijms25147633. Int J Mol Sci. 2024. PMID: 39062876 Free PMC article. Review.
-
Diagnostic Methods for Evaluation of Gastric Motility-A Mini Review.Diagnostics (Basel). 2023 Feb 20;13(4):803. doi: 10.3390/diagnostics13040803. Diagnostics (Basel). 2023. PMID: 36832289 Free PMC article. Review.
-
Comparing the efficacy of duloxetine and nortriptyline in alleviating the symptoms of functional dyspepsia - a randomized clinical trial.Front Psychiatry. 2024 Jan 16;14:1297231. doi: 10.3389/fpsyt.2023.1297231. eCollection 2023. Front Psychiatry. 2024. PMID: 38293596 Free PMC article.
References
-
- Andersson JLR, Skare S, Ashburner J (2003) How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. NeuroImage 20:870–888. - PubMed
-
- Badre D, Wagner AD (2007) Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia 45:2883–2901. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

