Inhibition of TRPC4 channel activity in colonic myocytes by tricyclic antidepressants disrupts colonic motility causing constipation
- PMID: 35560982
- PMCID: PMC9549500
- DOI: 10.1111/jcmm.17348
Inhibition of TRPC4 channel activity in colonic myocytes by tricyclic antidepressants disrupts colonic motility causing constipation
Abstract
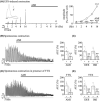
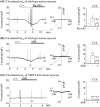
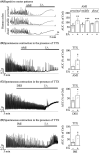
Tricyclic antidepressants (TCAs) have been used to treat depression and were recently approved for treating irritable bowel syndrome (IBS) patients with severe or refractory IBS symptoms. However, the molecular mechanism of TCA action in the gastrointestinal (GI) tract remains poorly understood. Transient receptor potential channel canonical type 4 (TRPC4), which is a Ca2+ -permeable nonselective cation channel, is a critical regulator of GI excitability. Herein, we investigated whether TCA modulates TRPC4 channel activity and which mechanism in colonic myocytes consequently causes constipation. To prove the clinical benefit in patients with diarrhoea caused by TCA treatment, we performed mechanical tension recording of repetitive motor pattern (RMP) in segment, electric field stimulation (EFS)-induced and spontaneous contractions in isolated muscle strips. From these recordings, we observed that all TCA compounds significantly inhibited contractions of colonic motility in human. To determine the contribution of TRPC4 to colonic motility, we measured the electrical activity of heterologous or endogenous TRPC4 by TCAs using the patch clamp technique in HEK293 cells and murine colonic myocytes. In TRPC4-overexpressed HEK cells, we observed TCA-evoked direct inhibition of TRPC4. Compared with TRPC4-knockout mice, we identified that muscarinic cationic current (mIcat ) was suppressed through TRPC4 inhibition by TCA in isolated murine colonic myocytes. Collectively, we suggest that TCA action is responsible for the inhibition of TRPC4 channels in colonic myocytes, ultimately causing constipation. These findings provide clinical insights into abnormal intestinal motility and medical interventions aimed at IBS therapy.
Keywords: TRPC4; irritable bowel syndrome; mIcat; smooth muscle; tricyclic antidepressant.
© 2022 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Pico145 inhibits TRPC4-mediated mICAT and postprandial small intestinal motility.Biomed Pharmacother. 2023 Dec;168:115672. doi: 10.1016/j.biopha.2023.115672. Epub 2023 Oct 17. Biomed Pharmacother. 2023. PMID: 37857250
-
Deletion of TRPC4 and TRPC6 in mice impairs smooth muscle contraction and intestinal motility in vivo.Gastroenterology. 2009 Oct;137(4):1415-24. doi: 10.1053/j.gastro.2009.06.046. Epub 2009 Jun 21. Gastroenterology. 2009. PMID: 19549525 Free PMC article.
-
Low concentrations of tricyclic antidepressants stimulate TRPC4 channel activity by acting as an opioid receptor ligand.Am J Physiol Cell Physiol. 2023 Jun 1;324(6):C1295-C1306. doi: 10.1152/ajpcell.00535.2022. Epub 2023 May 8. Am J Physiol Cell Physiol. 2023. PMID: 37154492
-
Molecular mechanisms of cholinergic neurotransmission in visceral smooth muscles with a focus on receptor-operated TRPC4 channel and impairment of gastrointestinal motility by general anaesthetics and anxiolytics.Neuropharmacology. 2024 Jan 1;242:109776. doi: 10.1016/j.neuropharm.2023.109776. Epub 2023 Oct 31. Neuropharmacology. 2024. PMID: 37913983 Review.
-
Direct modulation of TRPC ion channels by Gα proteins.Front Physiol. 2024 Feb 7;15:1362987. doi: 10.3389/fphys.2024.1362987. eCollection 2024. Front Physiol. 2024. PMID: 38384797 Free PMC article. Review.
Cited by
-
Prokinetic Activity of Mulberry Fruit, Morus alba L.Nutrients. 2023 Apr 14;15(8):1889. doi: 10.3390/nu15081889. Nutrients. 2023. PMID: 37111108 Free PMC article.
-
Atractylodes macrocephala Koidzumi modulates human colonic motility via ICCs pacemaker suppression and cAMP/ATP-sensitive K⁺ channel pathways.Int J Med Sci. 2025 Jul 24;22(13):3412-3421. doi: 10.7150/ijms.116169. eCollection 2025. Int J Med Sci. 2025. PMID: 40765567 Free PMC article.
-
Efficacy and Safety of Bacillus coagulans LBSC in Drug Induced Constipation Associated With Functional Gastrointestinal Disorder: A Double-Blind, Randomized, Interventional, Parallel, Controlled Trial a Clinical Study on Bacillus coagulans LBSC for Drug Induced Constipation Associated With FGIDs.Glob Adv Integr Med Health. 2024 Sep 17;13:27536130241286511. doi: 10.1177/27536130241286511. eCollection 2024 Jan-Dec. Glob Adv Integr Med Health. 2024. PMID: 39295947 Free PMC article.
-
Tricyclic antidepressants dose-dependently modulate the biphasic activity of the TRPC5 channel through opioid receptors.Korean J Physiol Pharmacol. 2025 Jul 1;29(4):455-464. doi: 10.4196/kjpp.25.121. Korean J Physiol Pharmacol. 2025. PMID: 40551090 Free PMC article.
-
Lubiprostone Improves Distal Segment-Specific Colonic Contractions through TRPC4 Activation Stimulated by EP3 Prostanoid Receptor.Pharmaceuticals (Basel). 2024 Oct 4;17(10):1327. doi: 10.3390/ph17101327. Pharmaceuticals (Basel). 2024. PMID: 39458968 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

