Analysis of Paediatric Clinical Trial Characteristics and Activity Over 23 Years-Impact of the European Paediatric Regulation on a Single French Clinical Research Center
- PMID: 35560985
- PMCID: PMC9086591
- DOI: 10.3389/fped.2022.842480
Analysis of Paediatric Clinical Trial Characteristics and Activity Over 23 Years-Impact of the European Paediatric Regulation on a Single French Clinical Research Center
Abstract
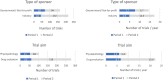
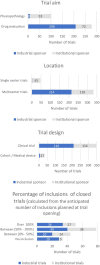
As unlicensed or off-label drugs are frequently prescribed in children, the European Pediatric Regulation came into force in 2007 to improve the safe use of medicinal products in the pediatric population. This present report analyzes the pediatric research trials over 23 years in a clinical research center dedicated to children and the impact of regulation. The database of trial characteristics from 1998 to 2020 was analyzed. We also searched for differences between two periods (1998-2006 and 2007-2020) and between institutional and industrial sponsors during the whole period (1998-2020). A total of 379 pediatric trials were initiated at our center, corresponding to inclusion of 7955 subjects and 19448 on-site patient visits. The trials were predominantly drug evaluation trials (n = 278, 73%), sponsored by industries (n = 216, 57%) or government/non-profit institutions (n = 163, 43%). All age groups and most subspecialties were concerned. We noted an important and regular increase in the number of trials conducted over the years, with an increased number of multinational, industrially sponsored trials. Based on the data presented, areas of improvement are discussed: (1) following ethical and regulatory approval depending on the sponsor, the mean time needed for administrative and financial agreement, validation of trial procedures allowing trial initiation at the level of the center was 6.3 and 6.5 months (periods 1 and 2, respectively) and should be reduced, (2) availability of expert research teams remain insufficient, time dedicated to research attributed to physicians should be organized and recognition of research nurses is required. The positive impact of the European Pediatric Regulation highlights the need to increase the availability of trained research teams, organized within identified multicenter international pediatric research networks.
Keywords: European paediatric regulation; drug evaluation; industrial trials; paediatric trial; public trials.
Copyright © 2022 Arnadottir, Luc, Kaguelidou, Jacqz-Aigrain and the Collaborative CIC1426 Investigator Group.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials

