Unmasking the suppressed immunopeptidome of EZH2-mutated diffuse large B-cell lymphomas through combination drug treatment
- PMID: 35561310
- PMCID: PMC9327544
- DOI: 10.1182/bloodadvances.2021006069
Unmasking the suppressed immunopeptidome of EZH2-mutated diffuse large B-cell lymphomas through combination drug treatment
Abstract
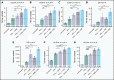
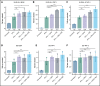
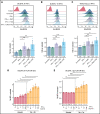
Exploring the repertoire of peptides presented on major histocompatibility complexes (MHCs) helps identify targets for immunotherapy in many hematologic malignancies. However, there is a paucity of such data for diffuse large B-cell lymphomas (DLBCLs), which might be explained by the profound downregulation of MHC expression in many DLBCLs, and in particular in the enhancer of zeste homolog 2 (EZH2)-mutated subgroup. Epigenetic drug treatment, especially in the context of interferon-γ (IFN-γ), restored MHC expression in DLBCL. In DLBCL, peptides presented on MHCs were identified via mass spectrometry after treatment with tazemetostat or decitabine alone or in combination with IFN-γ. Such treatment synergistically increased the expression of MHC class I surface proteins up to 50-fold and the expression of class II surface proteins up to threefold. Peptides presented on MHCs increased to a similar extent for both class I and class II MHCs. Overall, these treatments restored the diversity of the immunopeptidome to levels described in healthy B cells for 2 of 3 cell lines and allowed the systematic search for new targets for immunotherapy. Consequently, we identified multiple MHC ligands from the regulator of G protein signaling 13 (RGS13) and E2F transcription factor 8 (E2F8) on different MHC alleles, none of which have been described in healthy tissues and therefore represent tumor-specific MHC ligands that are unmasked only after drug treatment. Overall, our results show that EZH2 inhibition in combination with decitabine and IFN-γ can expand the repertoire of MHC ligands presented on DLBCLs by revealing suppressed epitopes, thus allowing the systematic analysis and identification of new potential immunotherapy targets.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures



Similar articles
-
Genome-wide Screens Identify Lineage- and Tumor-Specific Genes Modulating MHC-I- and MHC-II-Restricted Immunosurveillance of Human Lymphomas.Immunity. 2021 Jan 12;54(1):116-131.e10. doi: 10.1016/j.immuni.2020.11.002. Epub 2020 Dec 2. Immunity. 2021. PMID: 33271120 Free PMC article.
-
EZH2 Inhibition by Tazemetostat Results in Altered Dependency on B-cell Activation Signaling in DLBCL.Mol Cancer Ther. 2017 Nov;16(11):2586-2597. doi: 10.1158/1535-7163.MCT-16-0840. Epub 2017 Aug 23. Mol Cancer Ther. 2017. PMID: 28835384
-
Combined EZH2 Inhibition and IKAROS Degradation Leads to Enhanced Antitumor Activity in Diffuse Large B-cell Lymphoma.Clin Cancer Res. 2021 Oct 1;27(19):5401-5414. doi: 10.1158/1078-0432.CCR-20-4027. Clin Cancer Res. 2021. PMID: 34168051
-
Taking the EZ way: Targeting enhancer of zeste homolog 2 in B-cell lymphomas.Blood Rev. 2022 Nov;56:100988. doi: 10.1016/j.blre.2022.100988. Epub 2022 Jul 9. Blood Rev. 2022. PMID: 35851487 Free PMC article. Review.
-
Emerging EZH2 Inhibitors and Their Application in Lymphoma.Curr Hematol Malig Rep. 2018 Oct;13(5):369-382. doi: 10.1007/s11899-018-0466-6. Curr Hematol Malig Rep. 2018. PMID: 30112706 Review.
Cited by
-
Epigenetic modulation and prostate cancer: Paving the way for NK cell anti-tumor immunity.Front Immunol. 2023 Apr 5;14:1152572. doi: 10.3389/fimmu.2023.1152572. eCollection 2023. Front Immunol. 2023. PMID: 37090711 Free PMC article. Review.
-
Single-cell transcriptomic and spatial analysis reveal the immunosuppressive microenvironment in relapsed/refractory angioimmunoblastic T-cell lymphoma.Blood Cancer J. 2024 Dec 18;14(1):218. doi: 10.1038/s41408-024-01199-0. Blood Cancer J. 2024. PMID: 39695118 Free PMC article.
-
Harnessing the Molecular Fingerprints of B Cell Lymphoma for Precision Therapy.J Clin Med. 2022 Oct 1;11(19):5834. doi: 10.3390/jcm11195834. J Clin Med. 2022. PMID: 36233702 Free PMC article.
-
Therapeutic targeting of PRAME with mTCRCAR T cells in acute myeloid leukemia.Blood Adv. 2023 Apr 11;7(7):1178-1189. doi: 10.1182/bloodadvances.2022008304. Blood Adv. 2023. PMID: 35984639 Free PMC article.
-
Comprehensive analysis of UPK3B as a marker for prognosis and immunity in pancreatic adenocarcinoma.Sci Rep. 2025 Apr 13;15(1):12716. doi: 10.1038/s41598-025-97213-x. Sci Rep. 2025. PMID: 40223017 Free PMC article.
References
-
- Feugier P, Van Hoof A, Sebban C, et al. . Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol. 2005;23(18):4117-4126. - PubMed
-
- Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol. 2018;18(3):153-167. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

