Iptacopan monotherapy in patients with paroxysmal nocturnal hemoglobinuria: a 2-cohort open-label proof-of-concept study
- PMID: 35561315
- PMCID: PMC9636331
- DOI: 10.1182/bloodadvances.2022006960
Iptacopan monotherapy in patients with paroxysmal nocturnal hemoglobinuria: a 2-cohort open-label proof-of-concept study
Abstract
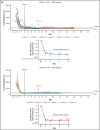
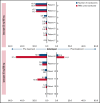
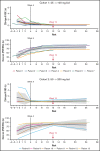
Iptacopan (LNP023) is a novel, oral selective inhibitor of complement factor B under clinical development for paroxysmal nocturnal hemoglobinuria (PNH). In this ongoing open-label phase 2 study, PNH patients with active hemolysis were randomized to receive single-agent iptacopan twice daily at a dose of either 25 mg for 4 weeks followed by 100 mg for up to 2 years (cohort 1) or 50 mg for 4 weeks followed by 200 mg for up to 2 years (cohort 2). At the time of interim analysis, of 13 PNH patients enrolled, all 12 evaluable for efficacy achieved the primary endpoint of reduction in serum lactate dehydrogenase (LDH) levels by ≥60% by week 12 compared with baseline; mean LDH levels dropped rapidly and durably, namely by 77% and 85% at week 2 and by 86% and 86% at week 12 in cohorts 1 and 2, respectively. Most patients achieved a clinically meaningful improvement in hemoglobin (Hb) levels, and all but 1 patient remained transfusion-free up to week 12. Other markers of hemolysis, including bilirubin, reticulocytes, and haptoglobin, showed consistent improvements. No thromboembolic events were reported, and iptacopan was well tolerated, with no severe or serious adverse events reported until the data cutoff. In addition to the previously reported beneficial effect of iptacopan add-on therapy to eculizumab, this study showed that iptacopan monotherapy in treatment-naïve PNH patients resulted in normalization of hemolytic markers and rapid transfusion-free improvement of Hb levels in most patients. This trial was registered at www.clinicaltrials.gov as #NCT03896152.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures




References
-
- Oni SB, Osunkoya BO, Luzzatto L. Paroxysmal nocturnal hemoglobinuria: evidence for monoclonal origin of abnormal red cells. Blood. 1970;36(2):145–152. - PubMed
-
- Kelly RJ, Hill A, Arnold LM, et al. Long-term treatment with eculizumab in paroxysmal nocturnal hemoglobinuria: sustained efficacy and improved survival. Blood. 2011;117(25):6786–6792. - PubMed
-
- Thomas TC, Rollins SA, Rother RP, et al. Inhibition of complement activity by humanized anti-C5 antibody and single-chain Fv. Mol Immunol. 1996;33(17-18):1389–1401. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

