Diversity of two-pore channels and the accessory NAADP receptors in intracellular Ca2+ signaling
- PMID: 35561646
- PMCID: PMC9645597
- DOI: 10.1016/j.ceca.2022.102594
Diversity of two-pore channels and the accessory NAADP receptors in intracellular Ca2+ signaling
Abstract
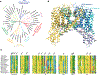
Intracellular Ca2+ signaling via changes or oscillation in cytosolic Ca2+ concentration controls almost every aspect of cellular function and physiological processes, such as gene transcription, cell motility and proliferation, muscle contraction, and learning and memory. Two-pore channels (TPCs) are a class of eukaryotic cation channels involved in intracellular Ca2+ signaling, likely present in a multitude of organisms from unicellular organisms to mammals. Accumulated evidence indicates that TPCs play a critical role in Ca2+ mobilization from intracellular stores mediated by the second messenger molecule, nicotinic acid adenine dinucleotide phosphate (NAADP). In recent years, significant progress has been made regarding our understanding of the structures and function of TPCs, including Cryo-EM structure determination of mammalian TPCs and characterization of a plastid TPC in a single-celled parasite.. The recent identification of Lsm12 and JPT2 as NAADP-binding proteins provides a new molecular basis for understanding NAADP-evoked Ca2+ signaling. In this review, we summarize basic structural and functional aspects of TPCs and highlight the most recent studies on the newly discovered TPC in a parasitic protozoan and the NAADP-binding proteins LSM12 and JPT2 as new key players in NAADP signaling.
Keywords: Calcium mobilization; Endolysosome; JPT2; Lsm12; Lysosome; NAADP; Two-pore channels.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest
The authors declare no competing interests.
Figures
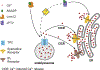
Similar articles
-
NAADP-binding proteins find their identity.Trends Biochem Sci. 2022 Mar;47(3):235-249. doi: 10.1016/j.tibs.2021.10.008. Epub 2021 Nov 20. Trends Biochem Sci. 2022. PMID: 34810081 Free PMC article. Review.
-
Convergent activation of two-pore channels mediated by the NAADP-binding proteins JPT2 and LSM12.Sci Signal. 2023 Aug 22;16(799):eadg0485. doi: 10.1126/scisignal.adg0485. Epub 2023 Aug 22. Sci Signal. 2023. PMID: 37607218 Free PMC article.
-
NAADP as an intracellular messenger regulating lysosomal calcium-release channels.Biochem Soc Trans. 2010 Dec;38(6):1424-31. doi: 10.1042/BST0381424. Biochem Soc Trans. 2010. PMID: 21118101
-
Lsm12 is an NAADP receptor and a two-pore channel regulatory protein required for calcium mobilization from acidic organelles.Nat Commun. 2021 Aug 6;12(1):4739. doi: 10.1038/s41467-021-24735-z. Nat Commun. 2021. PMID: 34362892 Free PMC article.
-
NAADP receptors.Cold Spring Harb Perspect Biol. 2011 Jan 1;3(1):a004036. doi: 10.1101/cshperspect.a004036. Cold Spring Harb Perspect Biol. 2011. PMID: 21047915 Free PMC article. Review.
Cited by
-
NAADP-Mediated Ca2+ Signalling.Handb Exp Pharmacol. 2023;278:3-34. doi: 10.1007/164_2022_607. Handb Exp Pharmacol. 2023. PMID: 35879580
-
In silico characterization of the gating and selectivity mechanism of the human TPC2 cation channel.J Gen Physiol. 2025 May 5;157(3):e202313506. doi: 10.1085/jgp.202313506. Epub 2025 Feb 21. J Gen Physiol. 2025. PMID: 39982432 Free PMC article.
References
-
- Patel S, NAADP-induced Ca2+ release – a new signalling pathway, Biol. Cell. 96 (2004) 19–28. - PubMed
-
- Santella L, NAADP: a new second messenger comes of age, Mol. Interv. 5 (2005) 70–72. - PubMed
-
- Cancela JM, Churchill GC, Galione A, Coordination of agonist-induced Ca2+-signalling patterns by NAADP in pancreatic acinar cells, Nature 398 (1999) 74–76. - PubMed
-
- Lee HC, Aarhus R, A derivative of NADP mobilizes calcium stores insensitive to inositol trisphosphate and cyclic ADP-ribose, J. Biol. Chem. 270 (1995) 2152–2157. - PubMed
-
- Aarhus R, Dickey DM, Graeff RM, Gee KR, Walseth TF, Lee HC, Activation and inactivation of Ca2+ release by NAADP+, J. Biol. Chem. 271 (1996) 8513–8516. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

