Topical therapy for regression and melanoma prevention of congenital giant nevi
- PMID: 35561684
- PMCID: PMC9237838
- DOI: 10.1016/j.cell.2022.04.025
Topical therapy for regression and melanoma prevention of congenital giant nevi
Abstract
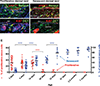
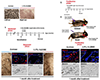
Giant congenital melanocytic nevi are NRAS-driven proliferations that may cover up to 80% of the body surface. Their most dangerous consequence is progression to melanoma. This risk often triggers preemptive extensive surgical excisions in childhood, producing severe lifelong challenges. We have presented preclinical models, including multiple genetically engineered mice and xenografted human lesions, which enabled testing locally applied pharmacologic agents to avoid surgery. The murine models permitted the identification of proliferative versus senescent nevus phases and treatments targeting both. These nevi recapitulated the histologic and molecular features of human giant congenital nevi, including the risk of melanoma transformation. Cutaneously delivered MEK, PI3K, and c-KIT inhibitors or proinflammatory squaric acid dibutylester (SADBE) achieved major regressions. SADBE triggered innate immunity that ablated detectable nevocytes, fully prevented melanoma, and regressed human giant nevus xenografts. These findings reveal nevus mechanistic vulnerabilities and suggest opportunities for topical interventions that may alter the therapeutic options for children with congenital giant nevi.
Keywords: Nras; congenital melanocytic nevus; hapten; melanoma; mole; prevention; topical.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests D.E.F. has a financial interest in Soltego, a company developing salt inducible kinase inhibitors for topical skin-darkening treatments that might be used for a broad set of human applications. The interests of D.E.F. were reviewed and are managed by Massachusetts General Hospital and Partners HealthCare in accordance with their conflict-of-interest policies. C.L.C. has a financial interest in 4Immune, a company developing cell therapy treatments that can be used for a broad set of human applications. The interests of C.L.C were reviewed and are managed by Mass General Brigham in accordance with their conflict-of-interest policies. X.S.L. is a cofounder, board member, SAB member, and consultant of GV20 Oncotherapy and its subsidiaries; stockholder of BMY, TMO, WBA, ABT, ABBV, and JNJ; and received research funding from Takeda, Sanofi, Bristol Myers Squibb, and Novartis. M.C.M. discloses consulting relationship with Novartis, Advisory Board with BioCoz and Caliber ID, and author royalties with Wiley & Sons.
Figures





Comment in
-
Making a mouse out of a molehill: how precision modeling repurposes drugs for congenital giant nevi.Trends Cancer. 2022 Aug;8(8):626-628. doi: 10.1016/j.trecan.2022.06.004. Epub 2022 Jun 17. Trends Cancer. 2022. PMID: 35718707 Free PMC article.
-
Treatment for giant congenital nevi moves a step closer.Cell Res. 2022 Sep;32(9):799-800. doi: 10.1038/s41422-022-00691-1. Cell Res. 2022. PMID: 35799061 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

