Disrupting autorepression circuitry generates "open-loop lethality" to yield escape-resistant antiviral agents
- PMID: 35561685
- PMCID: PMC9097017
- DOI: 10.1016/j.cell.2022.04.022
Disrupting autorepression circuitry generates "open-loop lethality" to yield escape-resistant antiviral agents
Erratum in
-
Disrupting autorepression circuitry generates ''open-loop lethality'' to yield escape-resistant antiviral agents.Cell. 2023 Apr 27;186(9):2036-2037. doi: 10.1016/j.cell.2023.03.020. Cell. 2023. PMID: 37116472 Free PMC article. No abstract available.
Abstract
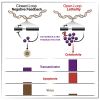
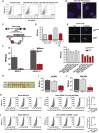
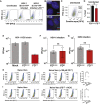
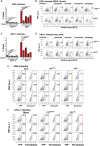
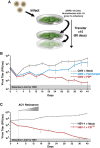
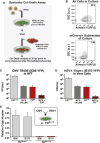
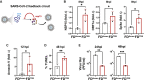
Across biological scales, gene-regulatory networks employ autorepression (negative feedback) to maintain homeostasis and minimize failure from aberrant expression. Here, we present a proof of concept that disrupting transcriptional negative feedback dysregulates viral gene expression to therapeutically inhibit replication and confers a high evolutionary barrier to resistance. We find that nucleic-acid decoys mimicking cis-regulatory sites act as "feedback disruptors," break homeostasis, and increase viral transcription factors to cytotoxic levels (termed "open-loop lethality"). Feedback disruptors against herpesviruses reduced viral replication >2-logs without activating innate immunity, showed sub-nM IC50, synergized with standard-of-care antivirals, and inhibited virus replication in mice. In contrast to approved antivirals where resistance rapidly emerged, no feedback-disruptor escape mutants evolved in long-term cultures. For SARS-CoV-2, disruption of a putative feedback circuit also generated open-loop lethality, reducing viral titers by >1-log. These results demonstrate that generating open-loop lethality, via negative-feedback disruption, may yield a class of antimicrobials with a high genetic barrier to resistance.
Keywords: autoregulatory circuit; feedback; nucleic acids; synthetic biology; transcriptional feedback; viral evolution.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests Competing interests: L.S.W. is an inventor of “Compositions and methods of use thereof for identifying antiviral agents” (US patent no. US10106817B2). L.S.W. and S.C. are inventors of “Compositions for herpesvirus transcriptional feedback circuit disruption and uses thereof” (US patent no. US2021/0395746A1).
Figures









Comment in
-
Cis-regulatory decoy disrupts autorepression: a potential escape-resistant anti-viral therapy.Signal Transduct Target Ther. 2022 Sep 27;7(1):338. doi: 10.1038/s41392-022-01187-5. Signal Transduct Target Ther. 2022. PMID: 36167795 Free PMC article. No abstract available.
References
-
- Alon U. Chapman & Hall/CRC; 2007. An introduction to systems biology: Design principles of biological circuits.
-
- Alon U., Surette M.G., Barkai N., Leibler S. Robustness in bacterial chemotaxis. Nature. 1999;397:168–171. - PubMed
-
- Aoki S.K., Lillacci G., Gupta A., Baumschlager A., Schweingruber D., Khammash M. A universal biomolecular integral feedback controller for robust perfect adaptation. Nature. 2019;570:533–537. - PubMed
-
- Arago, M. (1840). History of the steam engine, with a reply to the Criticisms to which the first publication of the article gave rise. Blasco de garay 1615, Solomon de Caus 1629. Sir Samuel Morland. Branca 1663. The Marquis of Worcester 1683. Denis Papin 1698, A.A. Lieut, and U.S.N. Harwood, translators 120 years B. C. Hiero of Alexandria 1543, p. 1690 and 1695. Captain Savery. (1705). Newcomen, Cawley, and Savery. Journal of the Franklin Institute, of the State of Pennsylvania, for the Promotion of the Mechanic Arts; Devoted to Mechanical and Physical Science, Civil Engineering, the Arts and Manufactures, and the Recording of American and Other Patent Inventions (1828-1851) 25, 3.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

