Translational development of a tumor junction opening technology
- PMID: 35562182
- PMCID: PMC9094124
- DOI: 10.1038/s41598-022-11843-z
Translational development of a tumor junction opening technology
Abstract
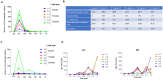
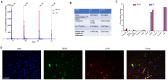
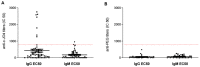
Our goal is to overcome treatment resistance in ovarian cancer patients which occurs in most cases after an initial positive response to chemotherapy. A central resistance mechanism is the maintenance of desmoglein-2 (DSG2) positive tight junctions between malignant cells that prevents drug penetration into the tumor. We have generated JO4, a recombinant protein that binds to DSG2 resulting in the transient opening of junctions in epithelial tumors. Here we present studies toward the clinical translation of c-JO4 in combination with PEGylated liposomal doxorubicin/Doxil for ovarian cancer therapy. A manufacturing process for cGMP compliant production of JO4 was developed resulting in c-JO4. GLP toxicology studies using material from this process in DSG2 transgenic mice and cynomolgus macaques showed no treatment-related toxicities after intravenous injection at doses reaching 24 mg/kg. Multiple cycles of intravenous c-JO4 plus Doxil (four cycles, 4 weeks apart, simulating the treatment regimen in the clinical trial) elicited antibodies against c-JO4 that increased with each cycle and were accompanied by elevation of pro-inflammatory cytokines IL-6 and TNFα. Pretreatment with steroids and cyclophosphamide reduced anti-c-JO4 antibody response and blunted cytokine release. Our data indicate acceptable safety of our new treatment approach if immune reactions are monitored and counteracted with appropriate immune suppression.
© 2022. The Author(s).
Conflict of interest statement
D.C. is founder of and holds an equity interest in HDT Bio Corp. a company that licensed the JO4 technology and is assisting in its clinical development.
Figures




References
-
- Tannock IF, Lee CM, Tunggal JK, Cowan DS, Egorin MJ. Limited penetration of anticancer drugs through tumor tissue: A potential cause of resistance of solid tumors to chemotherapy. Clin. Cancer Res. 2002;8:878–884. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

