Which cell death modality wins the contest for photodynamic therapy of cancer?
- PMID: 35562364
- PMCID: PMC9106666
- DOI: 10.1038/s41419-022-04851-4
Which cell death modality wins the contest for photodynamic therapy of cancer?
Abstract
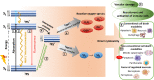
Photodynamic therapy (PDT) was discovered more than 100 years ago. Since then, many protocols and agents for PDT have been proposed for the treatment of several types of cancer. Traditionally, cell death induced by PDT was categorized into three types: apoptosis, cell death associated with autophagy, and necrosis. However, with the discovery of several other regulated cell death modalities in recent years, it has become clear that this is a rather simple understanding of the mechanisms of action of PDT. New observations revealed that cancer cells exposed to PDT can pass through various non-conventional cell death pathways, such as paraptosis, parthanatos, mitotic catastrophe, pyroptosis, necroptosis, and ferroptosis. Nowadays, immunogenic cell death (ICD) has become one of the most promising ways to eradicate tumor cells by activation of the T-cell adaptive immune response and induction of long-term immunological memory. ICD can be triggered by many anti-cancer treatment methods, including PDT. In this review, we critically discuss recent findings on the non-conventional cell death mechanisms triggered by PDT. Next, we emphasize the role and contribution of ICD in these PDT-induced non-conventional cell death modalities. Finally, we discuss the obstacles and propose several areas of research that will help to overcome these challenges and lead to the development of highly effective anti-cancer therapy based on PDT.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Raab O. Uber die Wirkung, fluorescirender Stoe auf infusorien. Z Biol. 1900;39:524–46.
-
- Tappeiner H, Jodlbauer A. Über die Wirkung der photodynamischen (fluorescierenden) Stoe auf Protozoen und Enzyme. Dtsch Arch Klin Med. 1904;39:427–87.
-
- Tappeiner H, Jodlbauer A. Die Sensibilisierende Wirkung fluorieszierender Substanzer. Gesammte Untersuchungen uber die photodynamische Erscheinung. Vogel, Leipzig: F. C. W; 1907.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

