Experimental research on surface acoustic wave microfluidic atomization for drug delivery
- PMID: 35562384
- PMCID: PMC9106708
- DOI: 10.1038/s41598-022-11132-9
Experimental research on surface acoustic wave microfluidic atomization for drug delivery
Abstract
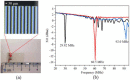
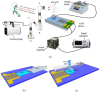
This paper demonstrates that surface acoustic wave (SAW) atomization can produce suitable aerosol concentration and size distribution for efficient inhaled lung drug delivery and is a potential atomization device for asthma treatment. Using the SAW device, we present comprehensive experimental results exploring the complexity of the acoustic atomization process and the influence of input power, device frequency, and liquid flow rate on aerosol size distribution. It is hoped that these studies will explain the mechanism of SAW atomization aerosol generation and how they can be controlled. The insights from the high-speed flow visualization studies reveal that it is possible by setting the input power above 4.17 W, thus allowing atomization to occur from a relatively thin film, forming dense, monodisperse aerosols. Moreover, we found that the aerosol droplet size can be effectively changed by adjusting the input power and liquid flow rate to change the film conditions. In this work, we proposed a method to realize drug atomization by a microfluidic channel. A SU-8 flow channel was prepared on the surface of a piezoelectric substrate by photolithography technology. Combined with the silicon dioxide coating process and PDMS process closed microfluidic channel was prepared, and continuous drug atomization was provided to improve the deposition efficiency of drug atomization by microfluidic.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Compact SAW aerosol generator.Biomed Microdevices. 2017 Mar;19(1):9. doi: 10.1007/s10544-017-0152-9. Biomed Microdevices. 2017. PMID: 28127655 Free PMC article.
-
Miniature inhalation therapy platform using surface acoustic wave microfluidic atomization.Lab Chip. 2009 Aug 7;9(15):2184-93. doi: 10.1039/b903575c. Epub 2009 May 14. Lab Chip. 2009. PMID: 19606295
-
Atomization off thin water films generated by high-frequency substrate wave vibrations.Phys Rev E Stat Nonlin Soft Matter Phys. 2012 Nov;86(5 Pt 2):056312. doi: 10.1103/PhysRevE.86.056312. Epub 2012 Nov 20. Phys Rev E Stat Nonlin Soft Matter Phys. 2012. PMID: 23214881
-
Surface acoustic wave atomizer and electrostatic deposition.Adv Biochem Eng Biotechnol. 2010;119:101-14. doi: 10.1007/10_2009_14. Adv Biochem Eng Biotechnol. 2010. PMID: 19826777 Review.
-
SAW-driven droplet jetting technology in microfluidic: A review.Biomicrofluidics. 2020 Dec 9;14(6):061505. doi: 10.1063/5.0014768. eCollection 2020 Nov. Biomicrofluidics. 2020. PMID: 33343781 Free PMC article. Review.
Cited by
-
Surface acoustic wave-based generation and transfer of droplets onto wettable substrates.RSC Adv. 2022 Aug 17;12(36):23400-23410. doi: 10.1039/d2ra04089a. eCollection 2022 Aug 16. RSC Adv. 2022. PMID: 36090390 Free PMC article.
-
Effect of the underlayer on the elastic parameters of the CoFeB/MgO heterostructures.Sci Rep. 2024 Aug 31;14(1):20259. doi: 10.1038/s41598-024-71110-1. Sci Rep. 2024. PMID: 39217241 Free PMC article.
-
Acoustofluidics: Technology Advances and Applications from 2022 to 2024.Anal Chem. 2025 Apr 8;97(13):6847-6870. doi: 10.1021/acs.analchem.4c06803. Epub 2025 Mar 25. Anal Chem. 2025. PMID: 40133046 Free PMC article. Review. No abstract available.
References
-
- Kwok PCL, McDonnell A, Tang P, Knight C, McKay E, Butler SP, Sivarajah A, Quinn R, Fincher L, Browne E, Yeo LY, Chan H. In vivo deposition study of a new generation nebuliser utilising hybrid resonant acoustic (HYDRA) technology. Int. J. Pharm. 2020;580:119196. doi: 10.1016/j.ijpharm.2020.119196. - DOI - PubMed
-
- Bodier-Montagutelli E, Morello E, L'Hostis G, Guillon A, Dalloneau E, Respaud R, Pallaoro N, Blois H, Vecellio L, Gabard J, Heuzé-Vourc'H N. Inhaled phage therapy: A promising and challenging approach to treat bacterial respiratory infections. Expert Opin. Drug Deliv. 2017;14:959–972. doi: 10.1080/17425247.2017.1252329. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

