MAIT cell compartment characteristics are associated with the immune response magnitude to the BNT162b2 mRNA anti-SARS-CoV-2 vaccine
- PMID: 35562666
- PMCID: PMC9100314
- DOI: 10.1186/s10020-022-00484-7
MAIT cell compartment characteristics are associated with the immune response magnitude to the BNT162b2 mRNA anti-SARS-CoV-2 vaccine
Abstract
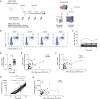
Mucosa-associated invariant T (MAIT) cells are unconventional T cells with innate-like capacity to rapidly respond to microbial infection via MR1-restricted antigen recognition. Emerging evidence indicate that they can also act as rapid sensors of viral infection via innate cytokine activation. However, their possible role in the immune response to mRNA vaccination is unknown. Here, we evaluated the involvement of MAIT cells in individuals vaccinated with the BNT162b2 mRNA SARS-CoV-2 vaccine. MAIT cell levels, phenotype and function in circulation were preserved and unperturbed through day 35 post-vaccination in healthy donor (HD) vaccinees, as well as people living with HIV (PLWH) or with primary immunodeficiency (PID). Unexpectedly, pre-vaccination and post-vaccination levels of MAIT cells correlated positively with the magnitude of the SARS-CoV-2 spike protein-specific CD4 T cell and antibody responses in the HD vaccinees. This pattern was largely preserved in the PID group, but less so in the PLWH group. Furthermore, in the HD vaccinees levels of MAIT cell activation and cytolytic potential correlated negatively to the adaptive antigen-specific immune responses. These findings indicate an unexpected association between MAIT cell compartment characteristics and the immune response magnitude to the BNT162b2 mRNA vaccine.
Keywords: Antibodies; BNT162b2; CD4 T cells; COVID-19; MAIT cells; SARS-CoV-2; mRNA vaccine.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing financial interests, patents, patent applications, or material transfer agreements associated with this study. M.B. is a consultant for Oxford Immunotec. S.A. has received honoraria for lectures and educational events, not related to this work, from Gilead, AbbVie, MSD, Biogen and Netdoktor.
Figures


References
-
- Bergman P, Blennow O, Hansson L, Mielke S, Nowak P, Chen P, et al. Safety and efficacy of the mRNA BNT162b2 vaccine against SARS-CoV-2 in five groups of immunocompromised patients and healthy controls in a prospective open-label clinical trial. EBioMedicine. 2021;74:103705. doi: 10.1016/j.ebiom.2021.103705. - DOI - PMC - PubMed
-
- Boulouis C, Gorin JB, Dias J, Bergman P, Leeansyah E, Sandberg JK. Opsonization-enhanced antigen presentation by MR1 activates rapid polyfunctional MAIT cell responses acting as an effector arm of humoral antibacterial immunity. J Immunol. 2020;205(1):67–77. doi: 10.4049/jimmunol.2000003. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

