VCP interaction with HMGB1 promotes hepatocellular carcinoma progression by activating the PI3K/AKT/mTOR pathway
- PMID: 35562734
- PMCID: PMC9102726
- DOI: 10.1186/s12967-022-03416-5
VCP interaction with HMGB1 promotes hepatocellular carcinoma progression by activating the PI3K/AKT/mTOR pathway
Abstract
Background: Hepatocellular carcinoma (HCC) is the most common pathological type of liver cancer. Valosin-containing protein (VCP) is a member of the AAA-ATPase family associated with multiple molecular functions and involved in tumor metastasis and prognosis. However, the role of VCP in HCC progression is still unclear.
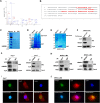
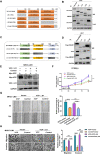
Methods: We examined the expression of VCP in HCC using the RNA sequencing and microarray data from public databases and measured it in clinical samples and cell lines by western blot, and immunohistochemistry (IHC). We also evaluated the correlation between VCP and clinical features. The VCP-interacting proteins were identified by co-immunoprecipitation combined with mass spectrometry (CoIP/MS). The underlying molecular mechanisms were investigated using in vitro and in vivo models of HCC.
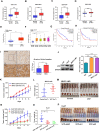
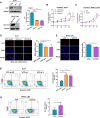
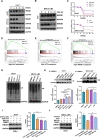
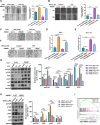
Results: We found that VCP expression is significantly increased in tumor tissues and is associated with advanced TNM stages and poorer prognosis in HCC patients. In vitro analyses revealed that VCP overexpression promoted HCC cell proliferation, migration, and invasion via PI3K/AKT/mTOR pathway activation. Conversely, VCP knockdown resulted in the reverse phenotypes. In vivo studies indicated that up-regulated VCP expression accelerated tumor growth in a subcutaneous HCC model. The D1 domain of VCP and A box of HMGB1 were identified as the critical regions for their interaction, and D1 area was required for the tumor-promoting effects induced by VCP expression. VCP enhanced the protein stability of HMGB1 by decreasing its degradation via ubiquitin-proteasome process. Inhibition of HMGB1 markedly attenuated VCP-mediated HCC progression and downstream activation of PI3K/AKT/mTOR signals.
Conclusion: Collectively, these findings demonstrate that VCP is a potential prognostic biomarker in HCC and exhibits oncogenic roles via PI3K/AKT/mTOR pathway activation. HMGB1 played an essential role in VCP-mediated HCC progression, indicating that VCP and HMGB1 are potential therapeutic targets in human HCC.
Keywords: HMGB1; Hepatocellular carcinoma; PI3K/AKT/mTOR pathway; Tumor progression; VCP.
© 2022. The Author(s).
Conflict of interest statement
Dr. Duda received consultant fees from Bayer, BMS, Simcere, Sophia Biosciences, and Surface Oncology and has received research grants from Bayer, Merrimack, Exelixis, and BMS. No reagents or support from these companies were used for this study. All the other authors declare that they have no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

