A type III effectiveness-implementation hybrid evaluation of a multicomponent patient navigation strategy for advanced-stage Kaposi's sarcoma: protocol
- PMID: 35562783
- PMCID: PMC9102240
- DOI: 10.1186/s43058-022-00281-7
A type III effectiveness-implementation hybrid evaluation of a multicomponent patient navigation strategy for advanced-stage Kaposi's sarcoma: protocol
Abstract
Background: For people with advanced-stage Kaposi's sarcoma (KS), a common HIV-associated malignancy in sub-Saharan Africa, mortality is estimated to be 45% within 2 years after KS diagnosis, despite increasingly wide-spread availability of antiretroviral therapy and chemotherapy. For advanced-stage KS, chemotherapy in addition to antiretroviral therapy improves outcomes and saves lives, but currently, only ~50% of people with KS in western Kenya who have an indication for chemotherapy actually receive it. This protocol describes the evaluation of a multicomponent patient navigation strategy that addresses common barriers to service penetration of and fidelity to evidence-based chemotherapy among people with advanced-stage KS in Kenya.
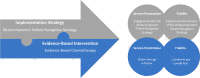
Methods: This is a hybrid type III effectiveness-implementation study using a non-randomized, pre- post-design nested within a longitudinal cohort. We will compare the delivery of evidence-based chemotherapy for advanced-stage KS during the period before (2016-2020) to the period after (2021-2024), the rollout of a multicomponent patient navigation strategy. The multicomponent patient navigation strategy was developed in a systematic process to address key determinants of service penetration of and fidelity to chemotherapy in western Kenya and includes (1) physical navigation and care coordination, (2) video-based education, (3) travel stipend, (4) health insurance enrollment assistance, (5) health insurance stipend, and (6) peer mentorship. We will compare the pre-navigation period to the post-navigation period to assess the impact of this multicomponent patient navigation strategy on (1) implementation outcomes: service penetration (chemotherapy initiation) and fidelity (chemotherapy completion) and (2) service and client outcomes: timeliness of cancer care, mortality, quality of life, stigma, and social support. We will also describe the implementation process and the determinants of implementation success for the multicomponent patient navigation strategy.
Discussion: This study addresses an urgent need for effective implementation strategies to improve the initiation and completion of evidence-based chemotherapy in advanced-stage KS. By using a clearly specified, theory-based implementation strategy and validated frameworks, this study will contribute to a more comprehensive understanding of how to improve cancer treatment in advanced-stage KS.
Keywords: Effectiveness-implementation hybrid; HIV-associated malignancies; Kaposi’s sarcoma; Low- and middle-income countries.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Impact of a multicomponent navigation strategy on stigma among people living with HIV and Kaposi's sarcoma in Kenya: a qualitative analysis.J Natl Cancer Inst Monogr. 2024 Jun 5;2024(63):38-44. doi: 10.1093/jncimonographs/lgae017. J Natl Cancer Inst Monogr. 2024. PMID: 38836529 Free PMC article.
-
Real-world use of chemotherapy for Kaposi's sarcoma in a large community-based HIV primary care system in Kenya.BMC Cancer. 2020 Jan 29;20(1):71. doi: 10.1186/s12885-019-6506-3. BMC Cancer. 2020. PMID: 31996161 Free PMC article.
-
Barriers and facilitators to chemotherapy initiation and adherence for patients with HIV-associated Kaposi's sarcoma in Kenya: a qualitative study.Infect Agent Cancer. 2022 Jul 6;17(1):37. doi: 10.1186/s13027-022-00444-0. Infect Agent Cancer. 2022. PMID: 35794634 Free PMC article.
-
Epidemiology of Kaposi's sarcoma in sub-Saharan Africa.Cancer Epidemiol. 2022 Jun;78:102167. doi: 10.1016/j.canep.2022.102167. Epub 2022 Apr 30. Cancer Epidemiol. 2022. PMID: 35504064 Review.
-
Facing up to the ongoing challenge of Kaposi's sarcoma.Curr Opin Infect Dis. 2015 Feb;28(1):31-40. doi: 10.1097/QCO.0000000000000122. Curr Opin Infect Dis. 2015. PMID: 25490104 Review.
Cited by
-
Impact of a multicomponent navigation strategy on stigma among people living with HIV and Kaposi's sarcoma in Kenya: a qualitative analysis.J Natl Cancer Inst Monogr. 2024 Jun 5;2024(63):38-44. doi: 10.1093/jncimonographs/lgae017. J Natl Cancer Inst Monogr. 2024. PMID: 38836529 Free PMC article.
-
Identification and development of implementation strategies: the important role of codesign.Front Health Serv. 2024 Feb 7;4:1305955. doi: 10.3389/frhs.2024.1305955. eCollection 2024. Front Health Serv. 2024. PMID: 38385048 Free PMC article.
-
Peer Navigation for HIV-Associated Kaposi Sarcoma in Kenya: The Power of the Survivor's Voice.JCO Glob Oncol. 2025 Mar;11:e2400494. doi: 10.1200/GO-24-00494. Epub 2025 Mar 20. JCO Glob Oncol. 2025. PMID: 40112257 Free PMC article.
-
Understanding referral of patients with cancer in rural Ethiopia: a qualitative study.BMC Cancer. 2024 May 2;24(1):553. doi: 10.1186/s12885-024-12294-7. BMC Cancer. 2024. PMID: 38698320 Free PMC article.
-
A case study of the development of a valid and pragmatic implementation science measure: the Barriers and Facilitators in Implementation of Task-Sharing Mental Health interventions (BeFITS-MH) measure.BMC Health Serv Res. 2024 Nov 6;24(1):1352. doi: 10.1186/s12913-024-11783-6. BMC Health Serv Res. 2024. PMID: 39501275 Free PMC article.
References
-
- Ferlay J, Laversanne M, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F. Global cancer observatory: cancer today. Lyon, France: International Agency for Research on Cancer; 2020.
-
- Mosam A, Shaik F, Uldrick TS, Esterhuizen T, Friedland GH, Scadden DT, Aboobaker J, Coovadia HM. A randomized controlled trial of highly active antiretroviral therapy versus highly active antiretroviral therapy and chemotherapy in therapy-naive patients with HIV-associated Kaposi sarcoma in South Africa. J Acquir Immune Defic Syndr. 2012;60(2):150–157. doi: 10.1097/QAI.0b013e318251aedd. - DOI - PMC - PubMed
-
- Semeere A, Wenger M, Busakhala N, Buziba N, Bwana M, Muyindike W, Amerson E, Maurer T, McCalmont T, LeBoit P, Musick B, Yiannoutsos C, Lukande R, Castelnuovo B, Laker-Oketta M, Kambugu A, Glidden D, Wools-Kaloustian K, Martin J. A prospective ascertainment of cancer incidence in sub-Saharan Africa: the case of Kaposi sarcoma. Cancer Med. 2016;5(5):914–928. doi: 10.1002/cam4.618. - DOI - PMC - PubMed