The development of a patient decision aid to reduce decisional conflict about antidepressant use in pregnancy
- PMID: 35562801
- PMCID: PMC9099318
- DOI: 10.1186/s12911-022-01870-1
The development of a patient decision aid to reduce decisional conflict about antidepressant use in pregnancy
Abstract
Background: People with moderate to severe depression in pregnancy must weigh potential risks of untreated or incompletely treated depression against the small, but uncertain risks of fetal antidepressant drug exposure. Clinical support alone appears insufficient for helping individuals with this complex decision. A patient decision aid (PDA) has the potential to be a useful tool for this population. The objective of our work was to use internationally recognized guidelines from the International Patient Decision Aids Standards Collaboration to develop an evidence-based PDA for antidepressant use in pregnancy.
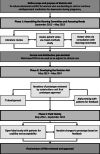
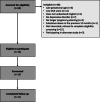
Methods: A three-phased development process was used whereby, informed by patient and physician perspectives and evidence synthesis, a steering committee commissioned a web-based PDA for those deciding whether or not to start or continue antidepressant treatment for depression in pregnancy (Phase 1). A prototype was developed (Phase 2) and iteratively revised based on feedback during field testing based on a user-centred process (Phase 3).
Results: We developed a web-based PDA for people deciding whether to start or continue antidepressant use for depression in pregnancy. It has five interactive sections: (1) information on depression and treatment; (2) reasons to start/continue an antidepressant and to start/stop antidepressant medication; (3) user assessment of values regarding each issue; (4) opportunity to reflect on factors that contribute to decision making; and (5) a printable PDF that summarizes the user's journey through the PDA.
Conclusions: This tool, which exclusively focuses on depression treatment with Selective Serotonin Reuptake Inhibitors and Serotonin-Norepinephrine Reuptake Inhibitors, can be used by individuals making decisions about antidepressant use to treat depression during pregnancy. Limitations of the PDA are that it is not for other conditions, nor other medications that can be used for depression, and in its pilot form cannot be used by women who do not speak English or who have a visual impairment. Pending further study, it has the potential to enhance quality of care and patient experience.
Keywords: Antidepressant; Depression; Online; Patient decision aid; Pregnancy.
© 2022. The Author(s).
Conflict of interest statement
SNV and DES report royalties from UpToDate for authorship of materials related to antidepressants in pregnancy. SG reports royalties from UpToDate and Psychotherapy Essentials to Go for authorship of materials related to treatment of depression in pregnancy. DES serves on the Medical Advisory Committee of Lilly’s Cymbalta Pregnancy Registry. The other authors have no conflicts of interest to declare.
Figures
References
-
- Jarde A, Morais M, Kingston D, Giallo R, MacQueen GM, Giglia L, et al. Neonatal outcomes in women with untreated antenatal depression compared with women without depression: A systematic review and meta-analysis. JAMA Psychiat. 2016;73(8):826–837. doi: 10.1001/jamapsychiatry.2016.0934. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources