Follicular helper T cell signature of replicative exhaustion, apoptosis, and senescence in common variable immunodeficiency
- PMID: 35562849
- PMCID: PMC9542315
- DOI: 10.1002/eji.202149480
Follicular helper T cell signature of replicative exhaustion, apoptosis, and senescence in common variable immunodeficiency
Erratum in
-
Correction.Eur J Immunol. 2023 Aug;53(8):e2370085. doi: 10.1002/eji.202370085. Eur J Immunol. 2023. PMID: 37565433 Free PMC article. No abstract available.
Abstract
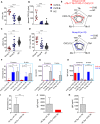
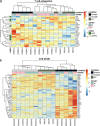
Common variable immunodeficiency (CVID) is the most frequent primary antibody deficiency whereby follicular helper T (Tfh) cells fail to establish productive responses with B cells in germinal centers. Here, we analyzed the frequency, phenotype, transcriptome, and function of circulating Tfh (cTfh) cells in CVID patients displaying autoimmunity as an additional phenotype. A group of patients showed a high frequency of cTfh1 cells and a prominent expression of PD-1 and ICOS as well as a cTfh mRNA signature consistent with highly activated, but exhausted, senescent, and apoptotic cells. Plasmatic CXCL13 levels were elevated in this group and positively correlated with cTfh1 cell frequency and PD-1 levels. Monoallelic variants in RTEL1, a telomere length- and DNA repair-related gene, were identified in four patients belonging to this group. Their blood lymphocytes showed shortened telomeres, while their cTfh were more prone to apoptosis. These data point toward a novel pathogenetic mechanism in CVID, whereby alterations in DNA repair and telomere elongation might predispose to antibody deficiency. A Th1, highly activated but exhausted and apoptotic cTfh phenotype was associated with this form of CVID.
Keywords: B cells; Common variable immunodeficiency; Immune aging; T follicular helper cells; T-cell exhaustion.
© 2022 The Authors. European Journal of Immunology published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no commercial or financial conflict of interest.
Figures







References
-
- Chapel, H. , Lucas, M. , Lee, M. , Bjorkander, J. , Webster, D. , Grimbacher, B. , Fieschi, C. et al., Common variable immunodeficiency disorders: division into distinct clinical phenotypes. Blood. 2008. 112: 277–286. - PubMed
-
- Fieschi, C. , Malphettes, M. and Galicier, L. Adult‐onset primary hypogammaglobulinemia. Presse. Med. 2006. 35: 887–894. - PubMed
-
- Gathmann, B. , Mahlaoui, N. , Gérard, L. , Oksenhendler, E. , Warnatz, K. , Schulze, I. , Kindle, G. et al., Clinical picture and treatment of 2212 patients with common variable immunodeficiency. J. Allergy. Clin. Immunol. 2014. 134: 117–137. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

